The Amendments to the IHR have been adopted
The 77th World Health Assembly HAS adopted a substantial package of amendments to the International Health Regulations. We the People have suffered a stunning defeat. The battle continues.
Unfortunately, this is an enormous loss for “We the People” and a substantial victory for the evil forces that support the system of pharmakia.
Please watch the videos below:
https://rumble.com/v4zbysk-pandemic-treaty-james-roguski-tpc-1499.html
The recently adopted amendments will facilitate an enormous global build up of the Pharmaceutical Hospital Emergency Industrial Complex which seeks to trigger ongoing “pandemic emergencies” that will be made even worse by “relevant health products.”
Article 13
The Director-General shall…
(c) support States Parties, upon their request, in scaling up and geographically diversifying the production of relevant health products,
Article 1
“relevant health products” means those health products needed to respond to public health emergencies of international concern, including pandemic emergencies, which may include medicines, vaccines, diagnostics, medical devices, vector control products, personal protective equipment, decontamination products, assistive products, antidotes, cell- and gene-based therapies, and other health technologies;
Article 44bis
1. A Coordinating Financial Mechanism (the Mechanism) is hereby established to:
Read the entire amended IHR:
https://apps.who.int/gb/ebwha/pdf_files/WHA77/A77_ACONF14-en.pdf
Please give your close attention to the following:
Article 1
Article 4
Article 12
Article 13
Article 24
Article 27
Article 31 (existing)
Article 35
Article 44
Article 44bis (not in bold text)
Article 54bis
Annex 1
Annex 4
Annex 6
Please watch the fraudulently conducted “vote” to adopt the amendments to the International Health Regulations:
https://rumble.com/v4yyrjt-the-amendments-to-the-ihr-have-been-adopted.html
Some important excerpts from the amendments:
Article 1
“National IHR Authority” means the entity designated or established by the State Party at the national level to coordinate the implementation of these Regulations within the jurisdiction of the State Party;
“pandemic emergency” means a public health emergency of international concern that is caused by a communicable disease and:
(i) has, or is at high risk of having, wide geographical spread to and within multiple States; and
(ii) is exceeding, or is at high risk of exceeding, the capacity of health systems to respond in those States; and
(iii) is causing, or is at high risk of causing, substantial social and/or economic disruption, including disruption to international traffic and trade; and
(iv) requires rapid, equitable and enhanced coordinated international action, with whole- of-government and whole-of-society approaches.
“relevant health products” means those health products needed to respond to public health emergencies of international concern, including pandemic emergencies, which may include medicines, vaccines, diagnostics, medical devices, vector control products, personal protective equipment, decontamination products, assistive products, antidotes, cell- and gene-based therapies, and other health technologies;
Article 4
1 bis. The National IHR Authority shall coordinate the implementation of these Regulations within the jurisdiction of the State Party.
Article 12
4 bis. If the Director-General determines that an event constitutes a public health emergency of international concern, the Director-General shall further determine, having considered the matters contained in paragraph 4, whether the public health emergency of international concern also constitutes a pandemic emergency.
Article 13
(c) support States Parties, upon their request, in scaling up and geographically diversifying the production of relevant health products,
(e) support States Parties, upon their request, and, as appropriate, through relevant WHO-coordinated and other networks and mechanisms, pursuant to subparagraph 8(c) of this Article, to promote research and development and strengthen local production of quality, safe and effective relevant health products,
Article 24
1. States Parties shall take all practicable measures consistent with these Regulations to ensure that conveyance operators:
(a) comply with the health measures recommended by WHO and adopted by the State Party, including for application on board as well as during embarkation and disembarkation;
(b) inform travellers of the health measures recommended by WHO and adopted by the State Party, including for application on board as well as during embarkation and disembarkation; and
Article 27
The competent authority may implement additional health measures, including isolation and quarantine of the conveyances, as necessary, to prevent the spread of disease. Such additional measures should be reported to the National IHR Focal Point.
Article 31 (existing)
The State Party may… compel the traveller to undergo…:
(a) the least invasive and intrusive medical examination that would achieve the public health objective;
(b) vaccination or other prophylaxis; or
(c) additional established health measures that prevent or control the spread of disease,
including isolation, quarantine or placing the traveller under public health observation.
Article 35
2. Health documents under these Regulations may be issued in non-digital format or digital format, subject to the obligations of any State Party regarding the format of such documents deriving from other international agreements.
3. Regardless of the format in which health documents under these Regulations have been issued, said health documents shall conform to the Annexes, referred to in Articles 36 to 39, as applicable, and their authenticity shall be ascertainable.
4. WHO, in consultation with States Parties, shall develop and update, as necessary, technical guidance, including specifications or standards related to the issuance and ascertainment of authenticity of health documents, both in digital format and non-digital format. Such specifications or standards shall be in accordance with Article 45 regarding treatment of personal data.
Article 44
2 bis. States Parties, subject to applicable law and available resources, shall maintain or increase domestic funding, as necessary, and collaborate, including through international cooperation and assistance, as appropriate, to strengthen sustainable financing to support the implementation of these Regulations.
2 ter. Pursuant to subparagraph (c) of paragraph 1, States Parties shall undertake to collaborate, to the extent possible, to:
(a) encourage governance and operating models of existing financing entities and funding mechanisms to be regionally representative and responsive to the needs and national priorities of developing countries in the implementation of these Regulations;
(b) identify and enable access to financial resources, including through the Coordinating Financial Mechanism, established pursuant to Article 44bis, necessary to equitably address the needs and priorities of developing countries, including for developing, strengthening and maintaining core capacities.
Article 44bis (not in bold text)
1. A Coordinating Financial Mechanism (the Mechanism) is hereby established to:
(a) promote the provision of timely, predictable, and sustainable financing for the implementation of these Regulations in order to develop, strengthen, and maintain core capacities as set out in Annex 1 of these Regulations, including those relevant for pandemic emergencies;
(b) seek to maximize the availability of financing for the implementation needs and priorities of States Parties, in particular of developing countries; and
(c) work to mobilize new and additional financial resources, and increase the efficient utilization of existing financing instruments, relevant to the effective implementation of these Regulations.
2. In support of the objectives set out in Paragraph 1 of this Article, the Mechanism shall, inter alia:
(a) use or conduct relevant needs and funding gap analyses;
(b) promote harmonization, coherence and coordination of existing financing instruments;
(c) identify all sources of financing that are available for implementation support and make this information available to States Parties;
(d) provide advice and support, upon request, to States Parties in identifying and applying for financial resources for strengthening core capacities, including those relevant for pandemic emergencies;
(e) leverage voluntary monetary contributions for organizations and other entities supporting States Parties to develop, strengthen and maintain their core capacities, including those relevant for pandemic emergencies.
3. The Mechanism shall function, in relation to the implementation of these Regulations, under the authority and guidance of the Health Assembly and be accountable to it.
Article 54 bis
1. The States Parties Committee for the Implementation of the International Health Regulations (2005) is hereby established to facilitate the effective implementation of these Regulations, in particular of Article 44 and 44bis.
4. The Committee shall adopt, at its first meeting, by consensus, terms of reference for the Coordinating Financial Mechanism, established in Article 44 bis, and modalities for its operationalization and governance and may adopt necessary working arrangements with relevant international bodies, which may support its operation as appropriate.
Annex 1
Each State Party shall develop, strengthen and maintain the core capacities:
(c) to coordinate with and support the Local level in preventing, preparing for and responding to public health risks and events, including in relation to:
(i) surveillance;
(ii) on-site investigations;
(iii) laboratory diagnostics, including referral of samples;
(iv) implementation of control measures;
(v) access to health services and health products needed for the response;
(vi) risk communication, including addressing misinformation and disinformation;
(vii) logistical assistance (e.g. equipment, medical and other relevant supplies and transport);
Annex 4
Section A Conveyance operators
1. Conveyance operators shall prepare for, as appropriate, and facilitate:(a) inspections of the cargo, containers and conveyance;
(b) medical examinations of persons on board;
(c) application of other health measures under these Regulations, including on board as well as during embarkation and disembarkation; and
(d) provision of relevant public health information requested by the State Party.
Annex 6
Regardless of the format in which they have been issued, certificates must bear the name of the clinician supervising the administration of the vaccine or prophylaxis, or of the relevant authority responsible for issuing the certificate or overseeing the administering centre.
8. For certificates under this Annex issued in non-digital format,
Aa parent or guardian shall sign the certificate when the child is unable to write. The signature of an illiterate A person who is unable to sign shall be indicated in the usual manner by the person’s mark and the indication by another that this is the mark of the person concerned, which shall be considered their signature. With respect to persons with a guardian, the guardian shall sign the certificate on their behalf.https://apps.who.int/gb/ebwha/pdf_files/WHA77/A77_ACONF14-en.pdf
Draft resolution proposed by France, Indonesia, Kenya, New Zealand, Saudi Arabia and the United States of America
(c) that future instruments on public health emergencies or pandemic prevention, preparedness and response, adopted pursuant to the WHO Constitution, may utilize the Coordinating Financial Mechanism contained in Article 44bis of the amended International Health Regulations (2005) to serve the implementation of such instruments;
https://apps.who.int/gb/ebwha/pdf_files/WHA77/A77_ACONF8Rev1-en.pdf
Compare to the IHR(2005):
https://iris.who.int/bitstream/handle/10665/246107/9789241580496-eng.pdf
Read the resolution:
https://apps.who.int/gb/ebwha/pdf_files/WHA77/A77_ACONF8-en.pdf
Watch the recordings of the World Health Assembly meetings here:
https://www.who.int/about/accountability/governance/world-health-assembly/seventy-seventh
Download all the WHA77 documents here:
https://apps.who.int/gb/e/e_wha77.html
The negotiations regarding the proposed “Pandemic Agreement” have been extended. The next negotiation session is scheduled for July 2024 (exact date TBD):
https://apps.who.int/gb/ebwha/pdf_files/WHA77/A77_ACONF15-en.pdf
For those who believe that the adoption of these amendments is somehow a “victory” for health freedom or that the amendments that were adopted “aren’t that bad” or that they “could have been worse,” or that we “dodged a number of bullets,” please realize that over the past year, quite a lot of misinformation has been spread regarding these amendments.
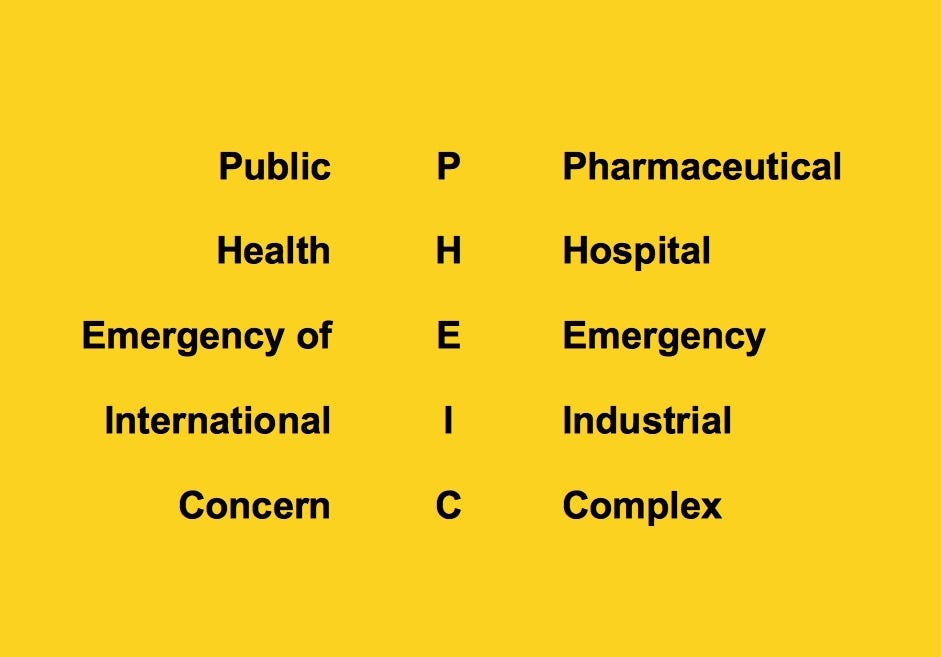
The build-up of the Pharmaceutical Hospital Emergency Industrial Complex that these amendments seek to implement in support of the equitable distribution of “relevant medical products” is certainly NOT a “victory” that should be celebrated.
Stop allowing yourself to be deceived.
ExitTheWHO.com
ExitTheWHO.org
PREVIOUS ARTICLES:
WARNING: GRAPHIC CONTENT
https://www.bitchute.com/video/AoTuksjRTBsN/
https://www.bitchute.com/video/kyQa5QLLJqhi/
James Roguski
The old system is crumbling, and we must build its replacement quickly.
If you are fed up with the government, hospital, medical, pharmaceutical, media, industrial complex and would like to help build a holistic alternative to the WHO, then feel free to contact me directly anytime.
JamesRoguski.substack.com/about
JamesRoguski.substack.com/archive
310-619-3055
All content is free to all readers.
All support is deeply appreciated.






















Thank you James for keeping us so well informed about this most serious violation of freedoms. This is a dark day for humanity, and I, as others have expressed, will not comply with such tyranny.
Watch the bird flu fearporn notch up a few gears now!!