Secret Moneypox Meeting
Tedros Ghebreyesus, the Director-General of the WHO, and the Emergency Committee will be meeting in secret on Wednesday, August 14 to decide whether moneypox should be declared a PHEIC.
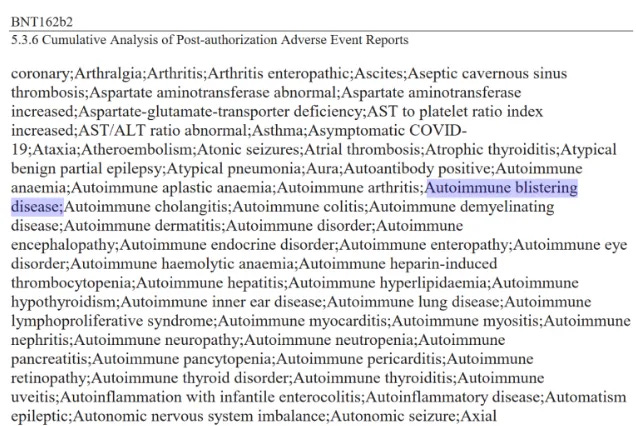
COVID-19 “vaccine” adverse events:
United States:
Public health authorities determine whether there is an mpox outbreak; a single case may be considered an mpox outbreak at the discretion of public health authorities.
Other circumstances in which a public health response may be indicated include ongoing risk of introduction of mpox into a community due to disease activity in another geographic area.
https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
Public health emergency as used in this part means:
(1) Any communicable disease event as determined by the Director with either documented or significant potential for regional, national, or international communicable disease spread or that is highly likely to cause death or serious illness if not properly controlled; or
(2) Any communicable disease event described in a declaration by the Secretary pursuant to 319(a) of the Public Health Service Act (42 U.S.C. 247d (a)); or
(3) Any communicable disease event the occurrence of which is notified to the World Health Organization, in accordance with Articles 6 and 7 of the International Health Regulations, as one that may constitute a Public Health Emergency of International Concern; or
(4) Any communicable disease event the occurrence of which is determined by the Director-General of the World Health Organization, in accordance with Article 12 of the International Health Regulations, to constitute a Public Health Emergency of International Concern; or
(5) Any communicable disease event for which the Director-General of the World Health Organization, in accordance with Articles 15 or 16 of the International Health Regulations, has issued temporary or standing recommendations for purposes of preventing or promptly detecting the occurrence or reoccurrence of the communicable disease.
https://www.govinfo.gov/content/pkg/FR-2017-01-19/pdf/2017-00615.pdf
The profiteering has already begun…
https://www.cdc.gov/poxvirus/mpox/clinicians/clinical-testing.html#tests
VACCINES
JYNNEOS is a third-generation vaccine based on a live, attenuated orthopoxvirus, Modified Vaccinia Ankara (MVA). MVA is a live virus that does not replicate efficiently in humans. JYNNEOS is known internationally as Imvamune® or Imvanex®; it is manufactured by Bavarian Nordic. It is fully licensed in the U.S. for subcutaneous administration in individuals 18 years of age and older. As of April 1, 2024, JYNNEOS is commercially available in the U.S.
In addition, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) in August 2022 to allow JYNNEOS vaccine to be used:
By intradermal injection for prevention of mpox disease in individuals 18 years of age and older determined to be at high risk for mpox, and
By subcutaneous injection for prevention of mpox disease in individuals younger than 18 years of age determined to be at high risk for mpox.
ACAM2000 is a second-generation vaccine that contains a live vaccinia virus that replicates efficiently in humans. It is manufactured by Emergent Bio Solutions and is indicated for the prevention of smallpox. It has been made available for use against mpox in the current outbreak under an Expanded Access Investigational New Drug (EA-IND) protocol, which requires informed consent along with completing additional forms. Available evidence supporting the use of smallpox vaccine for mpox prevention is derived from experience with Dryvax®, the vaccine used during smallpox eradication. Dryvax was a first-generation smallpox vaccine manufactured by Wyeth Laboratories. Routine use of this vaccine ended in the United States in 1972, and smallpox was declared eradicated globally in 1980 by the World Health Assembly. The license for Dryvax was withdrawn in 2008 and no supplies of this vaccine remain. Although the United States has a large supply of ACAM2000, this vaccine has more side effects and contraindications than JYNNEOS
https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
https://www.cdc.gov/poxvirus/mpox/vaccines/vaccine-recommendations.html
PHARMACEUTICAL DRUGS:
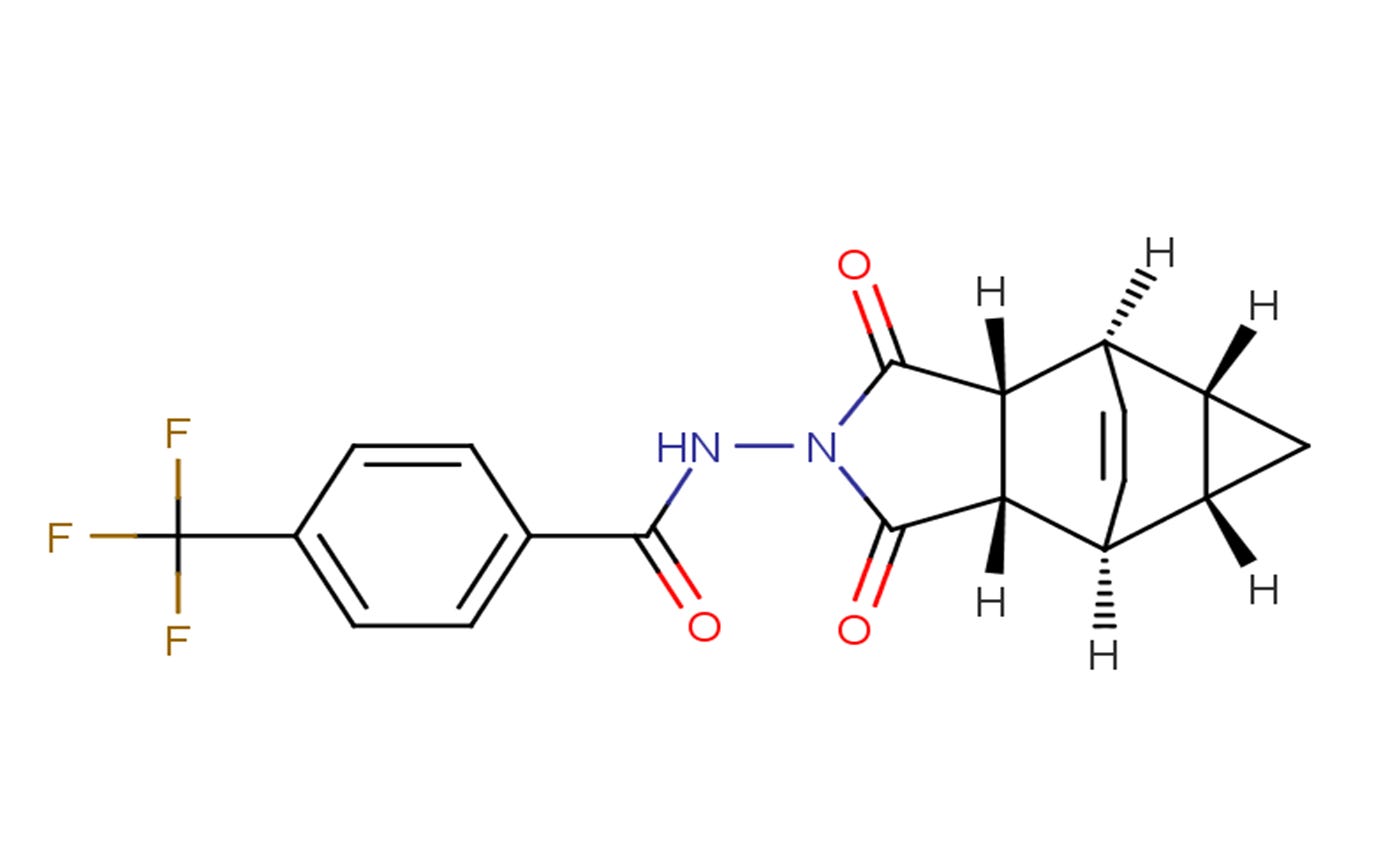
Tecovirimat: Because everyone needs more FLUORIDE.
CLICK HERE for side effects
https://www.cdc.gov/poxvirus/mpox/clinicians/tecovirimat-ea-ind.html
Please read this article…
James Roguski
310-619-3055
JamesRoguski.substack.com/archive
All content is free to all readers.
All support is deeply appreciated.






























Do not comply. We will not comply our way out of tyranny. They can’t arrest all of us! Onward.
thank you, James for continuing to sniff around the WHO's dirty laundry!!! Us common folk would be in the dark about their sadistic efforts without you. Stay Free!