REMDESIVIR
The government-hospital-medical-pharmaceutical-media industrial complex has been experimenting on our children for 22 months. THIS FRAUDULENT EXPERIMENT NEEDS TO STOP IMMEDIATELY!
Please watch the video below for an overview of this article.
TO MY READERS:
This article provides the information that the “powers that be” are conveniently ignoring and hiding from parents of children under 12 years of age.
For parents who are faced with the decision of giving their consent to allow their children to be injected with Veklury® (remdesivir), here’s the point:
Everyone under 12 years of age who is injected with Veklury® (remdesivir) is participating in an EXPERIMENT. The government and the medical establishment are breaking the law by not providing full disclosure on the risks of this EXPERIMENTAL treatment. PLEASE review all of the information in this article before allowing a doctor to give Veklury® (remdesivir) to your baby.
I am often criticized for publishing too much information (TMI). I fully realize that this is a long article. That’s because there is a lot of hidden information that needs to be revealed. I considered breaking it up into a series of articles, but I decided to publish it all in one place.
If you think it will take you too much time to read this article, consider how much time it took to research and write it. Please don’t complain that this article is too long. If this article saves one life, then it was worth writing.
Please read the entire article and then complain loudly at the people who have been keeping this enormous amount of information hidden from view.
James Roguski
310-619-3055
Please watch all the videos below…
1. Ali Schultz describes a new parent’s horror story.
2. David Knight summarizes the history of Veklury® (remdesivir) and raises the issue of data manipulation regarding the health of children.
3. Dr. Bryan Ardis is straightforward and to the point.
4. The World Health Organization recommends AGAINST the use of Veklury® (remdesivir).
EXPERT TESTIMONIES:
5. Dr. Paul Marik testifies about the toxic dangers of Veklury® (remdesivir) and the corruption in our medical system.
6. Frontline Nurse Nicole Sirotek's powerful testimony on how Veklury® (remdesivir) is killing patients.
7. Dr. Bryan Ardis testifies at the the Coronavirus Investigatory Committee’s Grand Jury proceedings regarding Veklury’s® (remdesivir’s) deadly outcomes.
SOURCES:
This information in this article was primarily obtained by simply reading the government and pharmaceutical company’s official documents and searching the Pub Med database of peer-reviewed published literature.
This information is publicly available on government websites for all to see.
I have merely read the documents below (so you don’t have to) and I extracted the information that parents should be given when they are considering the risks, both known and unknown, before consenting to allow their child to receive treatment with Veklury® (remdesivir).
Exhibit A. Original Letter of Authorization 4-1-2020
Exhibit B. Original EUA 4-16-2020
Exhibit C. Revised Letter of Authorization 10-16-2022
Exhibit D. EUA 10-16-2022
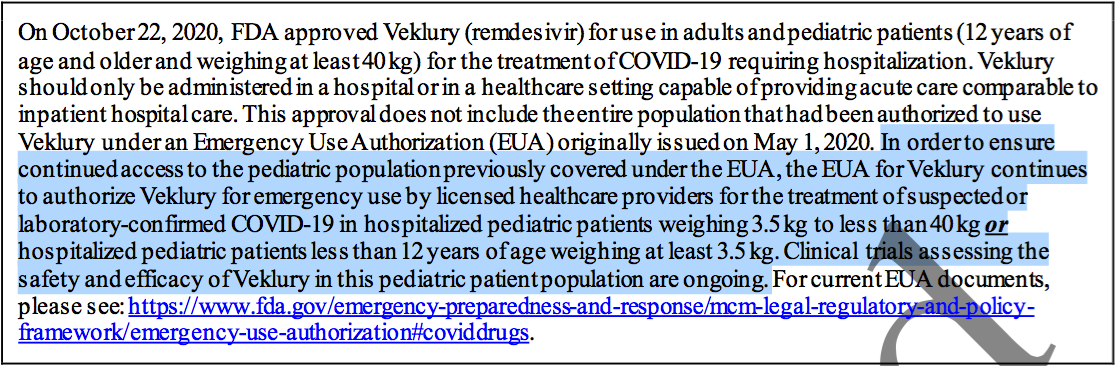
Exhibit E. Summary Review (10-21-2020)
Exhibit F. Approval Package (10-22-2020)
Exhibit G: Letter of Authorization 2-21-2022
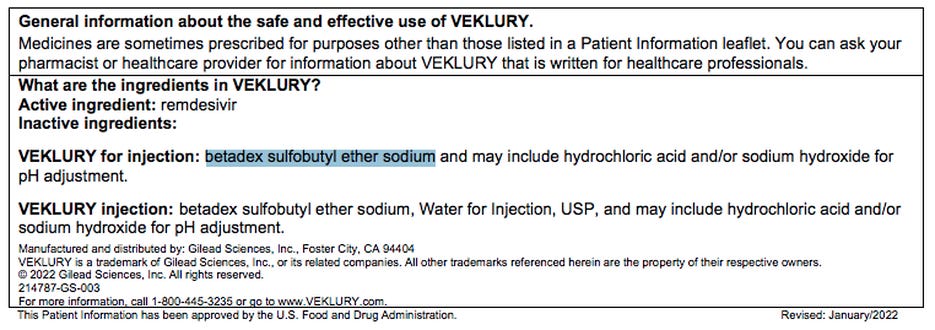
Exhibit H: Patient Information
Exhibit I: FACT SHEET for Health Care Providers
Exhibit J: FACT SHEET for Parents and Caregivers
Exhibit K: Prescribing Information
Exhibit L: FULL Prescribing Information
Exhibit M: Healthcare Provider Letter
Exhibit N: NIH Treatment Guidelines
Exhibit O: U.S. Code Emergency use of medical products
Exhibit P: MSDS Betadex Sulfobutyl Ether Sodium
Exhibit G: Website for Health Care Providers
Exhibit R: Website for Patients
Exhibit S: Important Facts Sheet
Exhibit T: NIAID website
Exhibit U: Public Health Emergency website
Exhibit V: New Covid Treatments Add-On Payments (NCTAP)
Exhibit W: Frequently Asked Questions
Exhibit X: Mechanism of Action and side effects (video)
Additional Exhibits: Multiple Peer-Reviewed Published Clinical Studies (see below)
OVERVIEW
In very simple terms, Veklury® (remdesivir) is only “approved” for adults and children OVER 12 years of age.
The use of Veklury® (remdesivir) in children UNDER 12 years of age is “authorized” for EXPERIMENTAL USE ONLY (hospitalized or outpatient).
Every parent who is asked to give their consent to the EXPERIMENTAL USE of Veklury® (remdesivir) should watch the video below IN ITS ENTIRETY.
CLICK HERE for a brief history of remdesivir:
The statements below cannot be denied. Anyone who denies these facts is spreading MDM.
Veklury® (remdesivir) has NOT been shown to be safe for children under 12 years of age.
Veklury® (remdesivir) has NOT been shown to be effective for children under 12 years of age.
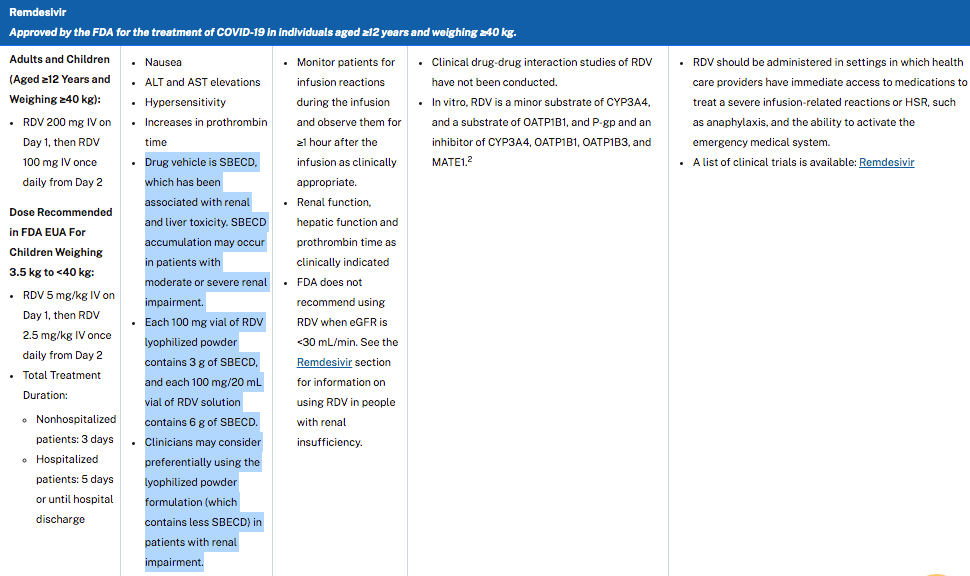
On January 21, 2022 the FDA’s acting Chief Scientist (Jacqueline A. O’Shaughnessy, Ph.D.) authorized (not approved) the experimental use of Veklury® (remdesivir) in children under 12 years of age weighing over 3.5kg on an outpatient basis.
The FDA’s letter of authorization clearly states: “No descriptive printed matter, advertising, or promotional materials relating to the use of Veklury® (remdesivir) under this authorization may represent or suggest that Veklury® (remdesivir) is safe or effective when used for the treatment of COVID-19.”
The Patient Information clearly states: “It is not known if Veklury® (remdesivir) is safe and effective in children under 12 years of age.”
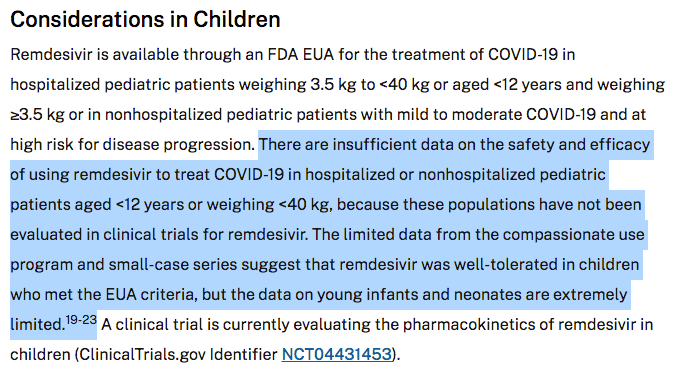
The NIH Treatment Guidelines clearly state that “there are insufficient data on the safety and efficacy of using remdesivir to treat COVID-19 in hospitalized pediatric patients aged <12 years or weighing <40kg because these population have not been evaluated in the clinical trials for remdesivir.”
The experimental use of Veklury® (remdesivir) in children has been ongoing since at least June 16, 2020 and so far NO results of this pharmacokinetics study have been made available to the public (as of February 28, 2022).
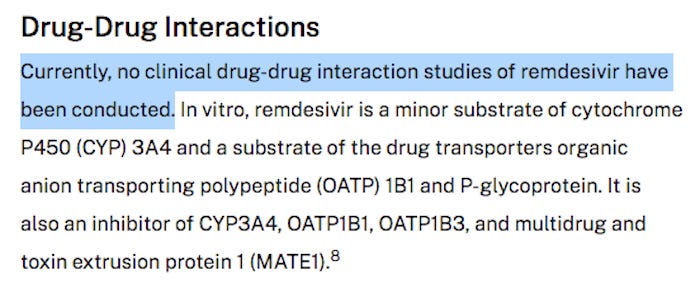
NO drug interaction studies have been completed, but a study published in July 2021 pointed out the potential for such drug interactions in people taking common drugs prescribed to treat their “co-morbidities.”
No dosage studies in children under 12 years of age were referenced in the EUA. The dosage for children was determined by “modeling and simulation.”
The Letter of Authorization failed to reference any studies that were done on anyone under 12 years of age. The data was “extrapolated” from studies done on MUCH older people.
The NIH Treatment Guidelines also acknowledge that the “inactive ingredient” betadex sulfobutyl ether sodium is known to be associated with kidney damage and that kidney function tests must be performed before using the drug.
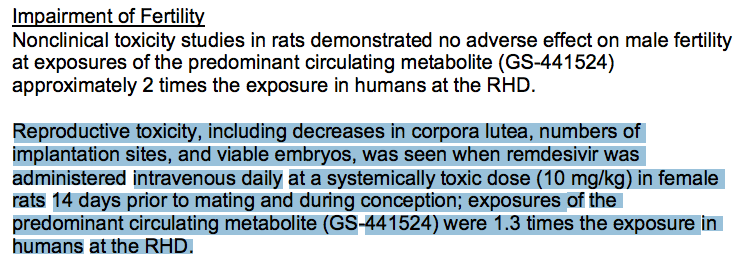
The FACT SHEET for Health Care Providers clearly states that studies have shown that “kidney-related effects in rats and monkeys were observed at exposures… that are lower than the exposure in humans at the recommended human dose (RHD).”
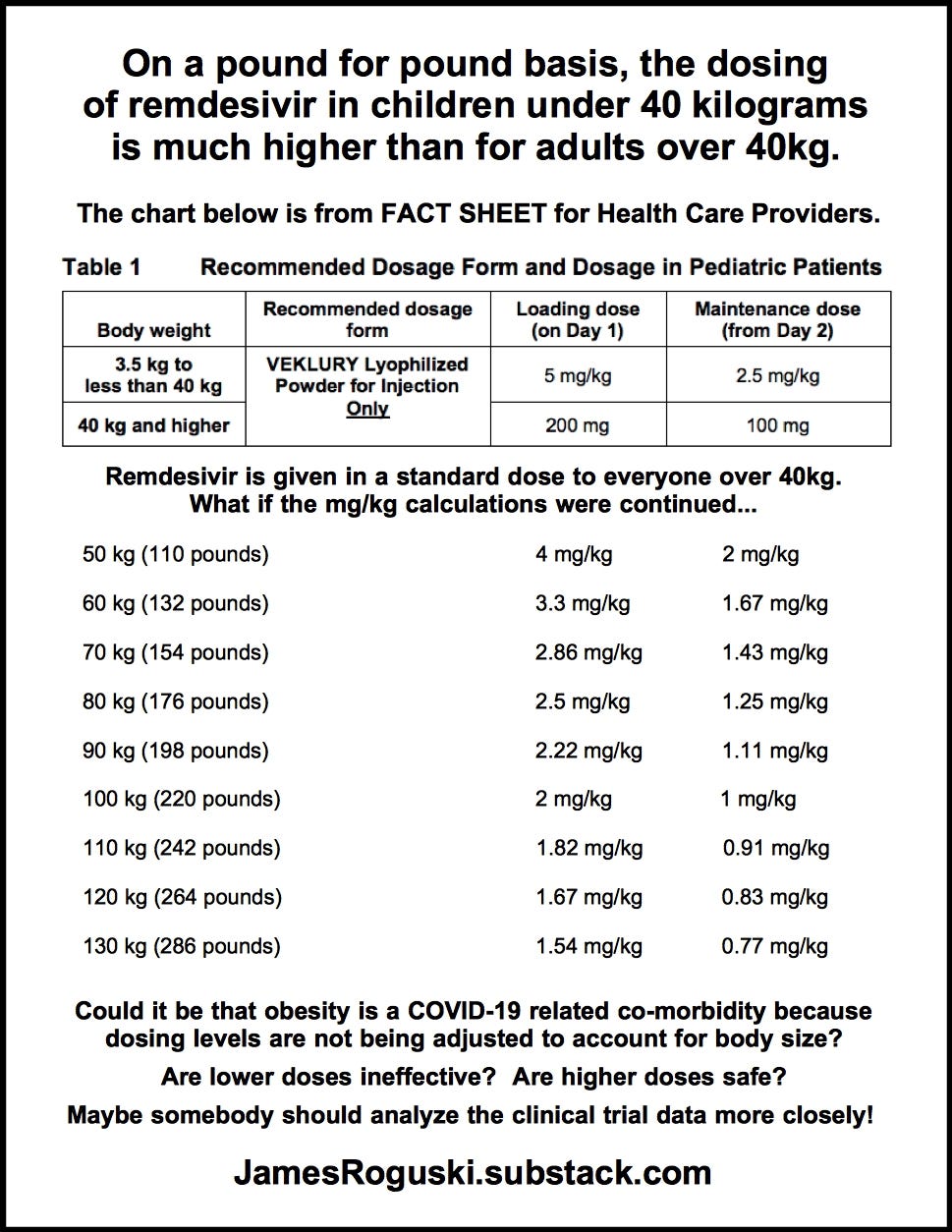
On a pound for pound basis, the recommended dosage for children who weigh less than 40 kilograms is actually TWICE the amount given to an adult of average weight (176 pounds).
A large number of peer-reviewed published studies (see below) show that Veklury® (remdesivir) is not effective and, in fact, is associated with kidney, liver and heart damage among many other negative outcomes including death.
In the information below, I have included screenshots of the important sections of the official documents. If you wish to research and verify the information further, you can click on the images to access the official documents. The page numbers are listed below the screenshots to help you find the specific information within each document.
The difference between “authorized” and “approved” centers around whether a drug, test or medical device is still “investigational/experimental” or has been accepted as being “safe and effective.”
If anyone ever says that a product or device which has been “authorized” for emergency use is “safe and effective,” you should immediately know that they are lying because, by definition, if it had met the criteria to be considered safe and effective, then it would have been “approved,” not just “authorized.”
The countless statements that have been made about remdesivir, the vaccines, RT-PCR tests (which are only authorized, not approved) and masks (which are unauthorized, unapproved medical devices being mandated by people practicing medicine without a license) are clearly and obviously lies.
The Letter of Authorization clearly states that “No descriptive printed matter, advertising, or promotional materials relating to the use of Veklury under this authorization may represent or suggest that Veklury is safe of effective when used for the treatment of COVID-19.”
The same information is repeated in the Patient Information document:
and in the Fact Sheet for Health Care Providers:
Veklury® (remdesivir) is still “investigational” in this age group.
Patients offered Veklury® (remdesivir) must be fully informed of all of the potential risks of the drug as well as its potential benefits before giving their consent. (See: 21 USC 360bbb-3a)
FDA documents clearly state that Veklury® (remdesivir) is not approved for use in children under the age of 12 years old. It is still in the “investigational” phase of the approval process.
Sufficiently powered (not enough participants) double blind placebo controlled studies that involved pediatric participants greater than 3.5kg and less than 40kg were NOT used to support the decision to authorize the use of Veklury (remdesivir) in pediatric patients. (See clinical trial details further below on this page.)
NOTE: The study mentioned above and shown below appears to be recruiting participants to study the use of Veklury® (remdesivir) in a very small number of infants but, as of February 24, 2022, no results have been published.
A Phase 2/3 Single-Arm, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734™) in Participants From Birth to < 18 Years of Age With COVID-19
https://www.clinicaltrials.gov/ct2/show/NCT04431453
The 4 studies that were referenced in the Letter Of Authorization (LOA) for Veklury® (remdesivir) did NOT include participants in the proper demographic/age group. The data used was “based on review and extrapolation of the data” from studies of much older individuals. It was not shown to be effective. Its effectiveness was merely “reasonable to believe.”
The dosage to be used in infants was not determined through clinical study, but was determined by “modeling and simulation.”
Is it safe to give infants a dose that is twice as much (by weight) as the approved dose for an adult that weighs 80kg?
Let me be perfectly clear: I am absolutely NOT advocating for higher doses of Veklury® (remdesivir).
Veklury® (remdesivir) has been associated with kidney failure, liver damage and unusual coagulation of the blood. In recognition of this, the following tests must be done PRIOR to administering Veklury® (remdesivir) AND during the course of treatment.
Veklury® (remdesivir) potently inhibits a natural human enzyme (CES2) that is found in the intestines, liver and kidneys and is important for detoxifying pharmaceutical medications.
Please watch the video below…
Remdesivir potently inhibits carboxylesterase-2 through covalent modifications: signifying strong drug-drug interactions
https://onlinelibrary.wiley.com/doi/epdf/10.1111/fcp.12643
CES2 is a major drug-metabolizing enzyme. The combination of high potency with irreversible inhibition concludes that cautions must be exercised when remdesivir is used along with drugs hydrolyzed by CES2.
The World Health Organization published a study that summarized the known pharmacokinetic properties of remdesivir on July 8, 2021.
ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential.
https://www.mdpi.com/1424-8247/14/7/655
https://www.mdpi.com/1424-8247/14/7/655/htm (full text)
I recognize the difference between a published paper that examines the theoretical potential for drug interactions versus pharmacokinetic studies that study and report on actual, real-world clinical results. However, in my humble opinion, the theoretical concerns raised by this paper should certainly have been mentioned in the official documents to alert attending physicians to the potential for suspected drug-drug interactions.
Did Gilead Sciences and the FDA simply not know about this published paper a full 6 months after it was published?
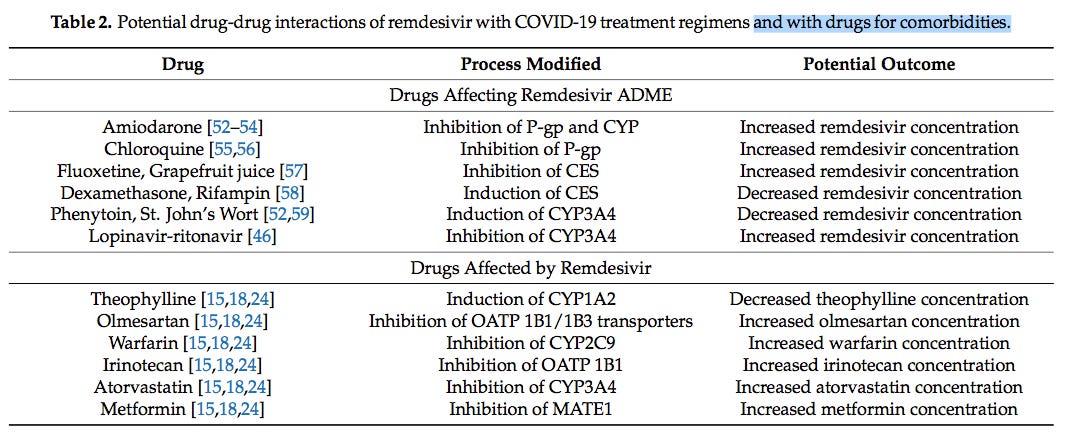
Or did Gilead Sciences and the FDA know about this published paper and willfully allow the theoretical potential for drug-drug interactions to remain hidden from public view by not including the following table in the official documents as a warning to attending physicians?
Is it possible that the well known connection between COVID-19 deaths and the risk factors commonly referred to as “co-morbidities” could be aggravated by drug interactions between Veklury® (remdesivir) and commonly used anti-coagulants and blood sugar, blood pressure and cholesterol lowering medications?
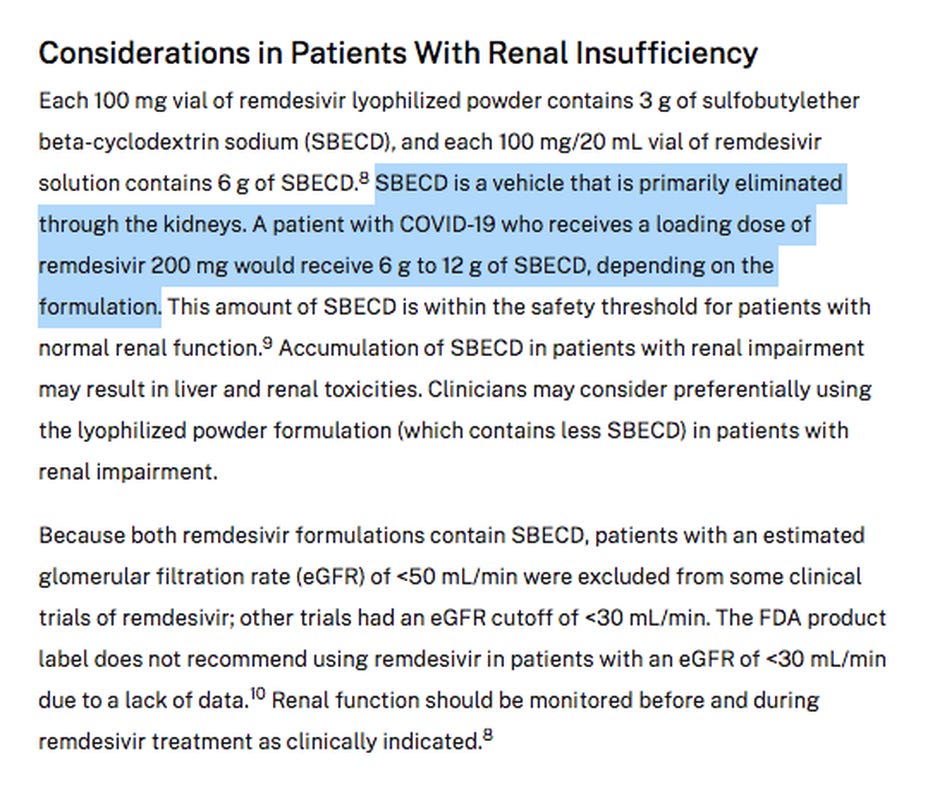
Both Veklury® (remdesivir) and the so-called “inactive ingredient” betadex sulfobutyl ether sodium (SBECD) are known to be associated with kidney damage. The NIH treatment protocols state “This amount of SBECD is within the safety threshold for patients with normal renal function.”
In toxicology, the average amount of a drug, toxin or chemical that is capable of killing 50% of the test animals is known as the Lethal Dose 50% (LD50).
In studies in rats, the LD50 for betadex sulfobutyl ether sodium is 2g/kg. If there was such a thing as a 40kg (88 pound) rat, it would take approximately 80 grams to cause half of the rats to die.
No placebo controlled studies were referenced in the EUA showing that betadex sulfobutyl ether sodium is safe to inject intravenously into American children under 12 years of age.
A full course of the FDA approved Veklury® (remdesivir) for an adult weighing 40kg will provide a total of 66g of betadex sulfobutyl ether sodium, which is dangerously close to 80g/40kg.
I realize that the red-capped version that was authorized for use in children has half the amount of betadex sulfobutyl ether sodium as stated in the example above.
The ONLY form of Veklury® (remdesivir) that has been authorized for experimental use in children under 12 yeasr of age is the powdered form in the vial with a red cap. It must be reconstituted, diluted and titrated to the proper amount based on the INFANT’S weight. The preparation process is quite complicated and prone to confusion and error because the dosage must be customized, by weight, for every patient.
The FDA chose to only authorize the red-capped version of Veklury® (remdesivir) for patients <40kg that contains 50% lower amounts of betadex sulfobutyl ether sodium.
While that appears to be a relatively safer choice, did the FDA make that decision because they recognized the toxicity of betadex sulfobutyl ether sodium and the kidney damage that it has been associated with?
The red-capped version of Veklury® (remdesivir) that is AUTHORIZED for emergency use in children under 12 years of age is different than the blue-capped version that is APPROVED for use in people over 12 years of age.
The red-capped version of Veklury® (remdesivir) and the blue-capped version are NOT interchangeable.
The blue capped version contains twice as much betadex sulfobutyl ether sodium (SBECD), but you would never know that because that information is not available on the label. I found it on the NIH website.
Chemical structure of betadex sulfobutyl ether sodium (SBECD)
New COVID-19 Treatments Add-On Payment (NCTAP)
The Centers for Medicare Services (CMS) issued an Interim Final Rule that established the New COVID-19 Treatments Add-on Payment (NCTAP). The NCTAP was designed incentivize hospitals to provide new COVID-19 treatments and went into effect on November 2, 2020. It will remain in effect until the end of the COVID-19 public health emergency (PHE).
The NCTAP provides an enhanced payment for eligible inpatient cases that use certain new products with current FDA approval or emergency use authorization (EUA) to treat COVID-19, including the following:
According to Wikipedia (not the greatest source), it costs 93¢ to produce a vial of Veklury® (remdesivir).
https://en.wikipedia.org/wiki/Remdesivir#Economics
The government price for Veklury® (remdesivir) is $390 per vial for federal entities that purchase the drug for use within their healthcare systems, such as the Veterans Health Administration, the Indian Health Service, the U.S. Coast Guard. All other entities, including American hospitals, will purchase VEKLURY® (remdesivir) at $520 per vial, to include vials used to treat Medicare and Medicaid patients.
Hospitalized (Severe): 11 doses over 10 days: 11 x $520= $5,720
Hospitalized (Moderate): 6 doses over 5 days: $3,120
Outpatient use: 4 doses over 3 days: $2080
Plus the 65% bonus for Veklury (remdesivir)
Plus the 20% bonus for the ENTIRE hospital bill for COVID-19 patients.
SOURCE:
https://www.cms.gov/medicare/covid-19/new-covid-19-treatments-add-payment-nctap
Without a realistic, honest and comprehensive risk/benefit analysis, it is absolutely impossible for any parent to give their “informed consent” on behalf of their child.
Neither the NIH, FDA nor the CDC have provided a comprehensive risk/benefit analysis for parents of children who weigh more than 3.5kg and less than 40kg.
The peer-reviewed published literature listed in FACT #10 below should be included as an integral part of the risk-benefit analysis to be reviewed by every person before they could possibly even consider giving their informed consent to treatment with Veklury@ (remdesivir).
The lack of a comprehensive risk/benefit analysis for the use of Veklury (remdesivir) as an investigational drug in children violates the laws regarding the fundamental right of refusal to grant fully informed consent to an experimental therapy.
NOTE: This information also applies to people who received Veklury® (remdesivir) prior to FDA approval on October 22, 2020.
21 USC § 50.20 General requirements for informed consent.
No investigator may involve a human being as a subject in research covered by these regulations unless the investigator has obtained the legally effective informed consent of the subject or the subject's legally authorized representative. An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence.
The information that is given to the subject or the representative shall be in language understandable to the subject or the representative.
No informed consent, whether oral or written, may include any exculpatory language through which the subject or the representative is made to waive or appear to waive any of the subject's legal rights, or releases or appears to release the investigator, the sponsor, the institution, or its agents from liability for negligence.
21 CFR § 50.25: Elements of informed consent.
(a) Basic elements of informed consent. In seeking informed consent, the following information shall be provided to each subject:
(1) A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures which are experimental.
(2) A description of any reasonably foreseeable risks or discomforts to the subject.
(3) A description of any benefits to the subject or to others which may reasonably be expected from the research.
(4) A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject.
(5) A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained and that notes the possibility that the Food and Drug Administration may inspect the records.
(6) For research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
(7) An explanation of whom to contact for answers to pertinent questions about the research and research subjects' rights, and whom to contact in the event of a research-related injury to the subject.
(8) A statement that participation is voluntary, that refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and that the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.
Compare the materials that are provided to patients with the explanation above of the information that is required by law.
It seems clear that a comprehensive risk/benefit analysis is not being provided to people who are asked to participate in clinical experiments.
It seems impossible for people to give their truly informed consent.
NOTE: I have been unable to locate/obtain the appropriate forms that should be given to people who choose (or give their consent on behalf of their children under the age of 12) to participate in the experimental use of Veklury® (remdesivir) under Emergency Use Authorization. The “IMPORTANT FACTS” document below is clearly inadequate.
None of the clinical trials referenced in the Emergency Use Authorization were conducted on children younger than 12 years old between 3.5kg and 40kg.
None of the studies were truly double-blinded.
Only two of the trials were placebo controlled. In one (ACTT-1), the clinical endpoints were changed in the middle of the trial. In the other, there was very little difference between the treatment group and the placebo group.
One of the trials merely compared different doses of Veklury (remdesivir). There was no comparison to a placebo.
One of the 4 trials was actually terminated early.
The 4 clinical trials referenced by the FDA in the Emergency Use Authorization are detailed below:
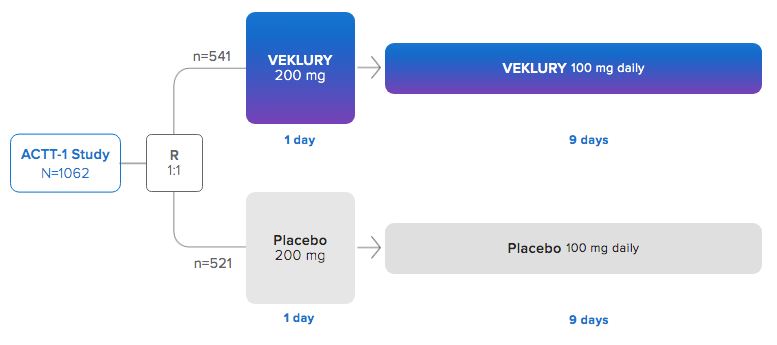
CLINICAL TRIAL #1
Adaptive COVID-19 Treatment Trial (ACTT-1) - It's all an ACTT.
https://clinicaltrials.gov/ct2/show/NCT04280705
The ACTT-1 study was a randomized, double-blind, placebo-controlled, phase 3 clinical trial in hospitalized adult patients with confirmed SARS-CoV-2 infection and mild, moderate, or severe COVID-19.
This clinical trial was one of two studies referenced in the initial EUA (May 1, 2020) even though the results were not publicly reported until September 25, 2020.
There are so many problems with this study, it boggles the mind.
Enrollment for ACTT-1 began on February 21, 2020, and ended on April 19, 2020.
The peer-reviewed paper reporting on this study was finally published in the New England Journal of Medicine on October 8, 2020.
Remdesivir for the Treatment of Covid-19 — Final Report
https://www.nejm.org/doi/full/10.1056/NEJMoa2007764
18 years of age and older - At baseline, mean age was 59 years
Last updated on December 9, 2020.
The final report shows that at least 35% of the patients were treated with hydroxychloroquine, probably with azithromycin. The data in the final report suggests that hydroxychloroquine, not remdesivir, was the main factor benefitting the patients in this study.
Nothing in the study supports the hypothesis that remdesivir is an effective antiviral for SARS-COV-2.
The study’s own numbers show an association between RDV and increased mortality in the most severe patients. It is also possible to conclude that RDV is net harmful for most hospitalized patients.
The study was not double blind, but was reported as such.
The pre-registered protocol was changed multiple times over the course of the trial and the primary outcome was changed in the middle of the trial, apparently because the researchers noticed a lack of effectiveness of their drug as tried
The study was marred with conflicts of interest, aggravated by the design giving NIAID and Gilead leverage over the hospitals and physicians treating patients At least three of the study researchers-authors failed to report grants and/or personal fees received from Gilead recently.
I STRONGLY RECOMMEND READING THIS ARTICLE: https://wattsupwiththat.com/2020/10/26/remdesivir-for-covid-19-study-accidentally-proved-effectiveness-of-hydroxychloroquine/
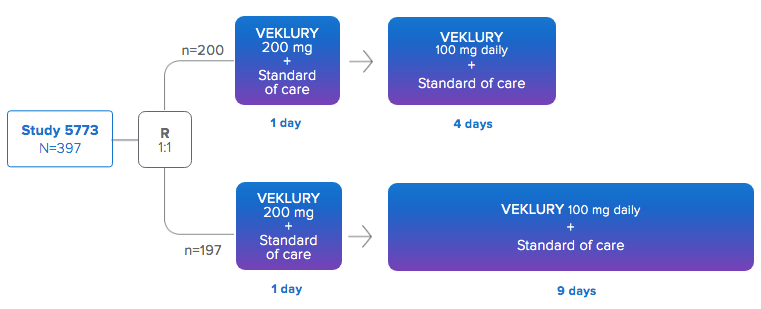
CLINICAL TRIAL #2
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19)
https://clinicaltrials.gov/ct2/show/NCT04292899
Study protocols: https://clinicaltrials.gov/ProvidedDocs/99/NCT04292899/Prot_000.pdf
AKA: Study GS-US-540-5773 in Hospitalized Subjects with Severe COVID-19
This clinical trial was one of two studies referenced in the initial EUA (May 1, 2020)
Study GS-US-540-5773 was a randomized, open-label, multicenter, phase 3 study in hospitalized adult patients with confirmed SARS-CoV-2 infection, an SpO2 of ≤94% on room air, or receiving supplemental oxygen and radiological evidence of pneumonia.
Last updated: December 31, 2020
This study was not blinded and there was no placebo group. It merely compared a 5 day course of remdesivir with a 10 day course. A greater perctenage of people died in the group that received 10 doses than those who received 5 doses.
“Overall, after adjusting for between-group differences at baseline, subjects receiving a 5-day course of VEKLURY had similar clinical status at Day 14 as those receiving a 10-day course (odds ratio for improvement 0.75 [95% CI 0.51 to 1.12]). There were no statistically significant differences in recovery rates or mortality rates in the 5-day and 10-day groups once adjusted for between-group differences at baseline. All-cause mortality at Day 28 was 12% vs 14% in the 5- and 10-day treatment groups, respectively.”
200 participants received treatment with remdesivir for 5 days
197 participants received treatment with remdesivir for 10 days
NO PLACEBO GROUP
12% - 14% OF THE TRIAL PARTICIPANTS DIED
12 years of age and older - At baseline, the median age of subjects was 61 years.
How could this STUDY possibly provide a rationale for approval in INFANTS?
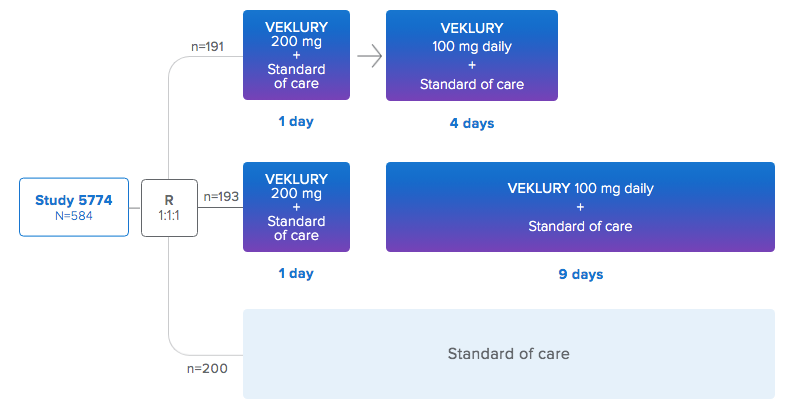
CLINICAL TRIAL #3
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment
https://clinicaltrials.gov/ct2/show/NCT04292730
Study Protocols: https://clinicaltrials.gov/ProvidedDocs/30/NCT04292730/Prot_000.pdf
AKA: Study GS-US-540-5774 in Hospitalized Subjects with Moderate COVID-19
Study GS-US-540-5774 was a randomized, open-label, multicenter, phase 3 study of hospitalized adult patients with confirmed SARS-CoV-2 infection, an SpO2 of >94% on room air, and radiological evidence of pneumonia.
191 participants received treatment with remdesivir for 5 days
193 participants received treatment with remdesivir for 10 days
200 received "Standard of Care"
Results:
“The odds of improvement in clinical status with the 10-day treatment group when compared to those receiving only standard of care were not statistically significant (odds ratio 1.31 [95% CI 0.88 to 1.95]). All-cause mortality at Day 28 was ≤2% in all treatment groups.”
12 years of age and older - At baseline, the median age of subjects was 57 years
How could this STUDY possibly provide a rationale for approval in INFANTS?
CLINICAL TRIAL #4
Study to Evaluate the Efficacy and Safety of Remdesivir (GS-5734™) Treatment of Coronavirus Disease 2019 (COVID-19) in an Outpatient Setting
https://clinicaltrials.gov/ct2/show/NCT04501952
AKA: Study GS-US-540-9012 in Non-Hospitalized Subjects with Mild-to-Moderate COVID-19 and at High Risk for Progression to Severe Disease
562 participants
279 participants received Veklury (remdesivir)
283 participants received placebo
“VEKLURY or placebo was first administered to subjects in outpatient facilities (84%), home healthcare settings (13%), or skilled nursing facilities (3%). The most common co-morbidities were diabetes mellitus (62%), obesity (56%), and hypertension (48%).”
12 years of age and older - At baseline, mean age was 50 years.
No one in either the treatment or placebo group died.
How could this STUDY possibly provide a rationale for approval in INFANTS?
5) Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results [Open label RCT]
https://pubmed.ncbi.nlm.nih.gov/33264556/
https://www.nejm.org/doi/pdf/10.1056/NEJMoa2023184
“Death occurred in 301 of 2743 patients receiving remdesivir and in 303 of 2708 receiving its control (rate ratio, 0.95; 95% confidence interval [CI], 0.81 to 1.11; P = 0.50)”
Based on the above study, the World Health Organization (WHO) has continuously recommended AGAINST the use of Veklury® remdesivir in patients of all age groups because there is no evidence that it improves survival.
Only 8 countries in the entire world have accepted the use of remdesivir for the treatment of COVID-19.
Australia
Brazil
Egypt
Ghana
India
Poland
United States
Venezuela
SOURCE:
The NIH, NIAID, FDA and the CDC have remained silent about many clinical studies that show Veklury® (remdesivir) is actually very harmful.
6) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext
http://clinicaltrials.gov/show/NCT04257656
Interpretation
In this study of adult patients admitted to hospital for severe COVID-19, remdesivir was not associated with statistically significant clinical benefits.
Wang et al. conducted a randomized, double-blind, placebo-controlled, multi-center study of 237 patients between February 6-March 12, 2020, in China. Their study did find evidence in support of using of Veklury® (remdesivir) to treat COVID-19.
The study found a non-statistically significant faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less.
In the study, patients were enrolled and randomly assigned to a treatment group (158 to remdesivir and 79 to placebo). Remdesivir use was not associated overall with a difference in time to clinical improvement.
Adverse events were reported in 102 (66%) of 155 remdesivir recipients versus 50 (64%) of 78 placebo recipients.
More patients in the remdesivir group than the placebo group suffered from respiratory failure or acute respiratory distress syndrome (10 % versus 8 %) and therefore discontinued the study drug (5 % versus 1 %).
Remdesivir was stopped early because of adverse events in 18 (12%) of the 158 remdesivir patients versus four placebo (5%).
More patients in the remdesivir group than the placebo group discontinued the study drug because of aminotransferase or bilirubin increases.
Overall, remdesivir was not associated with statistically significant clinical benefits against COVID-19 in their study and a majority of the remdesivir patients suffered adverse effects that were greater than those in the placebo group.
The authors also noted that remdesivir did not result in significant reductions in SARS-CoV-2 RNA loads or detectability in upper respiratory tract or sputum specimens, despite showing strong antiviral effects in preclinical models of infection with coronaviruses.
Additionally, the authors also noted that there were limitations of their study which included sufficient power to detect assumed differences in clinical outcomes and the initiation of remdesivir treatment quite late in COVID-19.
There was also no data on infectious virus recovery or on possible emergence of reduced susceptibility to remdesivir in viral isolates from these patients.
7) Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099%2821%2900485-0/fulltext
https://www.thelancet.com/action/showPdf?pii=S1473-3099%2821%2900485-0
Findings
“Between March 22, 2020, and Jan 21, 2021, 857 participants were enrolled and randomly assigned to remdesivir plus standard of care (n=429) or standard of care only (n=428). 15 participants were excluded from analysis in the remdesivir group, and ten in the control group. At day 15, the distribution of the WHO ordinal scale was: (1) not hospitalised, no limitations on activities (61 [15%] of 414 in the remdesivir group vs 73 [17%] of 418 in the control group); (2) not hospitalised, limitation on activities (129 [31%] vs 132 [32%]); (3) hospitalised, not requiring supplemental oxygen (50 [12%] vs 29 [7%]); (4) hospitalised, requiring supplemental oxygen (76 [18%] vs 67 [16%]); (5) hospitalised, on non-invasive ventilation or high flow oxygen devices (15 [4%] vs 14 [3%]); (6) hospitalised, on invasive mechanical ventilation or extracorporeal membrane oxygenation (62 [15%] vs 79 [19%]); (7) death (21 [5%] vs 24 [6%]).”
“The difference between treatment groups was not significant (odds ratio 0·98 [95% CI 0·77–1·25]; p=0·85). There was no significant difference in the occurrence of serious adverse events between treatment groups (remdesivir, 135 [33%] of 406 vs control, 130 [31%] of 418; p=0·48). Three deaths (acute respiratory distress syndrome, bacterial infection, and hepatorenal syndrome) were considered related to remdesivir by the investigators, but only one by the sponsor's safety team (hepatorenal syndrome).”
Interpretation
“No clinical benefit was observed from the use of remdesivir in patients who were admitted to hospital for COVID-19, were symptomatic for more than 7 days, and required oxygen support.”
8) Remdesivir for the Treatment of COVID-19: A Systematic Review of the Literature
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7390571/
“Until high-quality studies report significant improvements with administration of IV remdesivir, the use of this experimental drug should be limited to randomized controlled trials. Therefore, the potential of remdesivir as a standard of care therapy for COVID-19 remains to be determined.”
9) Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: A prospective randomised study [RCT]
https://europepmc.org/article/MED/33814589#free-full-text
Results:
“High-flow oxygen support and non-invasive ventilation was required at baseline by lesser patients in the remdesivir group. In the end, both groups had similar outcomes after adjustment for baseline clinical status. There was no statistical difference in mortality between the two groups (p = 0.749). Patients in both groups had an equal time to recovery. There was no difference in the occurrence of adverse effects of remdesivir between the two groups.”
Conclusion:
“Remdesivir therapy for five days did not produce improvement in clinical outcomes in moderate to severe COVID-19 cases.”
https://c19rmd.com/mahajan.html
34 people treated
76.5% higher risk of death, 111.8% higher risk of mechanical ventilation.
10) Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19 [Retrospective]
Conclusions and Relevance
“In this cohort study of US veterans hospitalized with COVID-19, remdesivir treatment was not associated with improved survival but was associated with longer hospital stays. Routine use of remdesivir may be associated with increased use of hospital beds while not being associated with improvements in survival.”
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2781959
1171 people treated
6% higher risk of death, 100% increase in hospitalization time
11) Efficacy of remdesivir in Japanese patients hospitalised with COVID-19: A large observational study using the COVID-19 Registry Japan [Retrospective]
Conclusions
“Remdesivir might reduce the risk of oxygen requirement during hospitalization in the early stage of COVID-19; however, it had no positive effect on the clinical outcome and reduction of IMV/ECMO requirement.”
https://www.ijidonline.com/article/S1201-9712(22)00118-7/fulltext
https://c19rmd.com/tsuzuki.html
74 people treated
8.8% lower risk of death, 17.1% increased risk of mechanical ventilation or ECMO, 27.3% increase in hospitalization time.
12) Efficacy of Remdesivir in Covid-19 Patients; Multicenter Study in Lahore [Late treatment study]
https://www.ijsciences.com/pub/pdf/V92020112417.pdf
Conclusion:
“There was no significant positive outcome found in patients who were administered intravenous Remdesivir in patients who required mechanical ventilation or not or whether they had any co-existing disease or not.”
30 people treated
100% higher risk of death, 250% higher risk of mechanical ventilation
13) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [RCT]
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2820%2931022-9
In summary, we found that this dose regimen of intravenous remdesivir was adequately tolerated but did not provide significant clinical or antiviral effects in seriously ill patients with COVID-19.
158 people treated
In patients treated early: 24.3 lower mortality
In patients treated later: 47.6% higher mortality
14) LiverTox: Clinical and Research Information on Drug-Induced Liver Injury.
https://www.ncbi.nlm.nih.gov/books/NBK564049/
“In the liver, remdesivir is metabolized by and is a substrate for CYP 3A4. It is also a substrate of the hepatocyte transporters p-glycoprotein and OATP1B1 and thus may be susceptible to drug-drug interactions with agents that inhibit or induce these enzymes and transporters.”
“Careful monitoring of liver tests is recommended before and frequently during treatment. However, this recommendation is difficult in outpatient situations when the drug is given for 3 days only. Nevertheless, it is recommended that de novo elevations in ALT or AST above 10 times the upper limit of normal should lead to consideration of early discontinuation of the infusions. Furthermore, development of symptoms of hepatitis or jaundice should lead to immediate discontinuation.”
“Use of other known hepatotoxins or potent inhibitors of p-glycoprotein should be avoided during remdesivir therapy.”
15) Liver injury in remdesivir-treated COVID-19 patients
“Our observation supports previous findings obtained in healthy volunteers (Gilead Sciences, data on file) and COVID-19 patients treated with RDV [4, 5], suggesting this antiviral may cause hepatocellular injury.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7386240/
16) Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database
https://pubmed.ncbi.nlm.nih.gov/33340409/
“Based on ARF cases reported in VigiBase, and despite the caveats inherent to COVID-19 circumstances, we detected a statistically significant pharmacovigilance signal of nephrotoxicity associated with remdesivir, deserving a thorough qualitative assessment of all available data.”
17) Kidney disorders as serious adverse drug reactions of remdesivir in coronavirus disease 2019: a retrospective case–noncase study
“Our findings, based on post marketing real-life data from >5000 COVID-19 patients, support that kidney disorders, almost exclusively AKI, represent a serious, early, and potentially fatal adverse drug reaction of remdesivir.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7907730/
18) Potential kidney damage associated with the use of remdesivir for COVID-19: analysis of a pharmacovigilance database
https://pubmed.ncbi.nlm.nih.gov/34787281/
“The available information on the renal safety profile for remdesivir is limited, thus we analyzed the renal and urinary adverse reactions attributed to remdesivir reported in a large open pharmacovigilance database. We obtained reports of remdesivir and other drugs used to treat COVID-19 (tocilizumab, hydroxychloroquine, lopinavir/ritonavir) registered by September 30 2020, from the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). We found 2,922 reports with remdesivir registered in FAERS for COVID-19. Among these, 493 renal and urinary adverse effects (16.9%) were reported. The most frequent events were acute kidney injury (338; 11.6%), renal impairment (86; 2.9%), and renal failure (53; 1.8%). Versus hydroxychloroquine, lopinavir/ritonavir, or tocilizumab, the use of remdesivir was associated with an increased chance of reporting renal and urinary disorders regardless of gender and age of patients (2.53; 95%CI: 2.10-3.06). Our results reinforce this already reported signal, emphasizing that it could be extremely useful for health professionals who prescribe this new antiviral to treat COVID-19, mainly knowing its low efficacy.”
19) Assessment of adverse events associated with remdesivir use for coronavirus disease 2019 using real-world data
https://pubmed.ncbi.nlm.nih.gov/34328807/
Results:
“Elevated liver enzymes, acute kidney injury, raised blood creatinine levels, bradycardia, cardiac arrest, and death had disproportionately higher reporting with remdesivir as asuspect drug compared with other drugs.”
20) [NON] Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials
https://pubmed.ncbi.nlm.nih.gov/35100985/
Results:
No antiviral treatment demonstrated efficacy at reducing COVID-19 mortality. Remdesivir exhibited efficacy in reducing time to recovery, but results were inconsistent across trials.
Conclusions:
Although select antivirals have exhibited efficacy to improve clinical outcomes in COVID-19 patients, none demonstrated efficacy in reducing mortality.
21) Cardiac Adverse Events With Remdesivir in COVID-19 Infection
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7682945/
Conclusions
“Some SARS-CoV-2 patients on remdesivir develop sinus bradycardia and a prolonged QT interval. Appropriate caution and continuous EKG monitoring should be utilized in all patients participating in ongoing trials for COVID-19 as the safety of remdesivir remains largely uncertain. Even closer surveillance for patients with pre-existing heart disease is warranted when using remdesivir.”
22) Safety profile of the antiviral drug remdesivir: An update
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7373689/
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7373689/table/tbl0005/?report=objectonly
Since most COVID-19 patients with liver injury had mild aminotransferases and/or bilirubin increases [7], if abnormality of liver enzymes occur after remdesivir initiation, especially in high levels, adverse drug reactions need to be considered and drug discontinuation is required if necessary.
23) Liver injury in remdesivir-treated COVID-19 patients
https://academic.oup.com/cid/article/71/15/756/5803302
https://link.springer.com/article/10.1007/s12072-020-10077-3
Our observation supports previous findings obtained in healthy volunteers (Gilead Sciences, data on file) and COVID-19 patients treated with RDV [4, 5], suggesting this antiviral may cause hepatocellular injury.
24) Remdesivir induces persistent mitochondrial and structural damage in human induced pluripotent stem cell derived cardiomyocytes
https://pubmed.ncbi.nlm.nih.gov/34609482/
Using an in vitro model, we demonstrated that remdesivir can induce cardiotoxicity in hiPSC-CMs at clinically relevant concentrations. These results reveal previously unknown potential side-effects of remdesivir and highlight the importance of further investigations with in vivo animal models and active clinical monitoring to prevent lasting cardiac damage to patients.
25) Complete heart block associated with Remdesivir in COVID-19: a case report
https://academic.oup.com/ehjcr/article/5/7/ytab200/6312606
https://doi.org/10.1093/ehjcr/ytab200
Within 24 h of starting Remdesivir, he was noted to be in atrial fibrillation with ventricular rates between 30 and 40 b.p.m. On Day 5 of Remdesivir therapy, ECG demonstrated complete AV block. Having completed the Remdesivir regimen, during the next 48 h, he was closely monitored, and the AV block resolved spontaneously.
Remdesivir was developed through an academic-corporate partnership between Gilead Sciences and the Baric Lab at the University of North Carolina at Chapel Hill’s Gillings School of Global Public Health. The biopharmaceutical company sought the talents of a research team led by Ralph Baric.
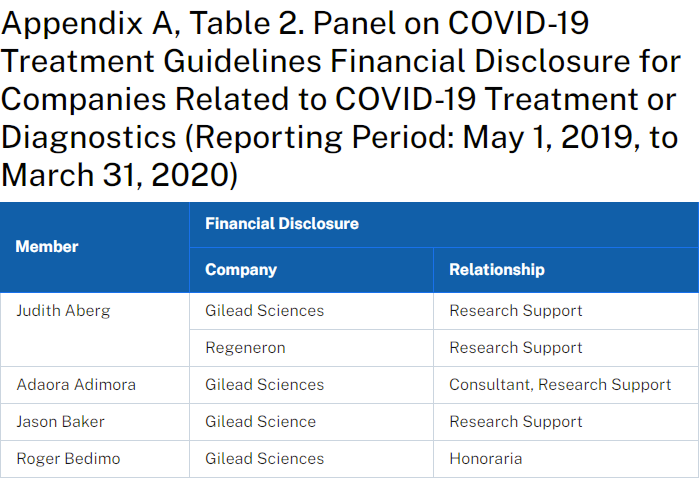
By using the Wayback Machine (web.archive.org) to view an archived page from April 21, 2020, it has been revealed that 9 members of the advisory committee that voted to approve the use of remdesivir had disclosed conflicts of interest with the manufacturer Gilead Sciences.
Current conflicts of interest:
https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/panel-financial-disclosure/
Additional Articles:
https://www.brightworkresearch.com/how-gilead-brought-off-the-nihs-support-of-remdesivir/
In October 2020, Veklury® (remdesivir) was approved by the FDA without much discussion.
If more than a week has passed since the onset of COVID-19 like symptoms, it is highly questionable for your doctor to recommend that Veklury® (remdesivir) to be administered.
Dr. Vladimir Zelenko’s protocols using hydroxychloroquine and zinc have been shown in numerous studies to be of benefit when given preventively as well as very early in the course of COVID-19 because it is in pill form and is relatively inexpensive.
Veklury® (remdesivir) costs hundreds of dollars per dose and requires 3 separate visits to an outpatient facility in order to receive the medication via intravenous infusion.
Please read my (soon to be published) article on the controversy involving Rick Bright, Janet Woodcock, hydroxychloroquine and remdesivir.
FDA is requiring health care providers who prescribe Veklury® (remdesivir) to pediatric patients under the EUA to report all serious adverse events and medication errors potentially related to Veklury® (remdesivir) through FDA’s MedWatch Adverse Event Reporting program. Providers can complete and submit the report online; or download and complete the form, then submit it via fax at 1-800-FDA-0178. A copy of the MedWatch form submitted to FDA should also be provided to Gilead.
However, there is NO requirement to report adverse events associated with the use of Veklury® (remdesivir) when it is used in patients over 12 years of age.
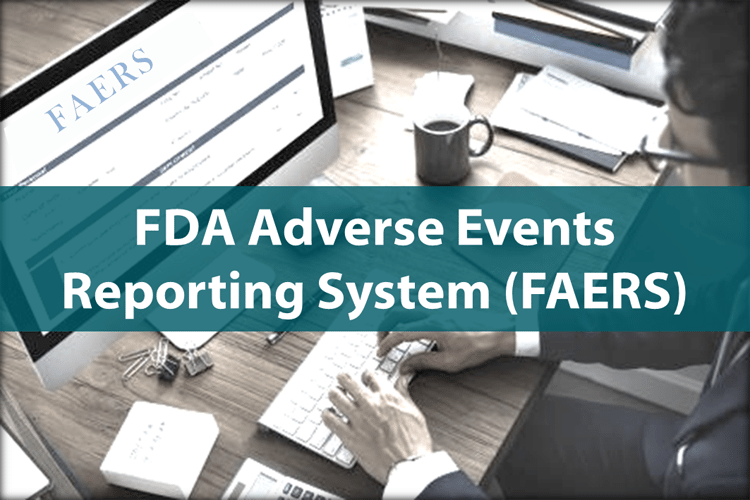
FAERS (not VAERS)
Did you think that there were issues associated with VAERS?
Have you ever heard of FAERS?
The FDA Adverse Event Reporting System (FAERS) is a computerized information database designed to support the U.S. Food and Drug Administration's (FDA) post marketing safety surveillance program for all approved drug and therapeutic biologic products.
The FDA uses FAERS to monitor for new adverse events and medication errors that might occur with these products. It is a system that measures occasional harms from medications to ascertain whether the risk–benefit ratio is high enough to justify continued use of any particular drug and to identify correctable and preventable problems in health care delivery (such as need for retraining to prevent prescribing errors). The system interacts with several related systems including MedWatch and the Vaccine Adverse Event Reporting System (VAERS).
If you thought VAERS was inadequate, click on the links below and check out FAERS!
https://open.fda.gov/data/faers/
https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html
FREEDOM OF INFORMATION ACT REQUESTS
These previously requested documents are readily available in the public domain via search engine inquiry.
From: 01-Jan-2010 To: 06-Oct-2020
From: 01-Jan-2020 To: 03-Nov-2020
From: 01-Feb-2020 To: 02-Jun-2020
From: 01-Apr-2020 To: 03-Aug-2020
IS THE COVID-19 PANDEMIC OVER?
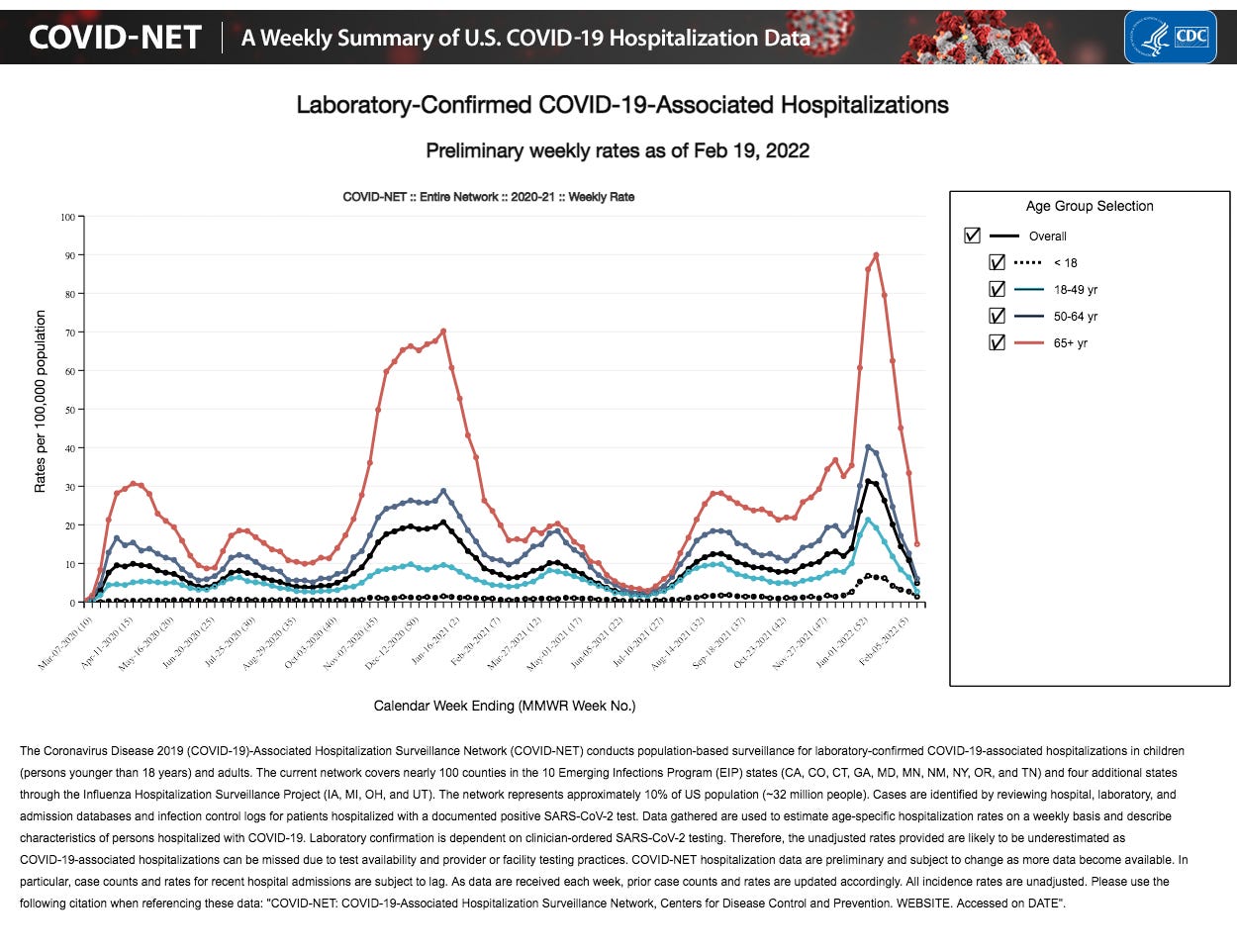
The chart below is from https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html
According to the chart above, it would appear that “the pandemic” is over. What is missing from the chart above is the number of “incidental” cases of COVID-19. An “incidental” case is one in which a person is hospitalized for some reason other than COVID-19, but due to financial incentives, they are found to “test positive” for COVID-19 and lumped into the above data, rather than be identified for what they really are - an ‘incidental” case.
How many “incidental” COVID-19 cases were administered Veklury® (remdesivir) in order to increase hospital revenue and improve the statistics in support of Veklury®’s efficacy at treating a diagnosed disease that didn’t really exist?
IMPORTANT MISSING/UNAVAILABLE DATA:
I hope to be able to update this article and include the data items listed below, but as of the initial publication date of this article, I have been unable to locate the following data:
Total number of COVID-19 hospitalized patients (nationwide)
Total number of “incidental” COVID-19 cases.
Total number of patients who have received Veklury® remdesivir
Hospitalized case fatality rates (Veklury®/not Veklury®)
Detailed demographic breakdown of actual dosages given and the associated outcomes and adverse events, including number of days since onset of symptoms.
Total funds paid out by the government to Gilead Sciences and to hospitals participating in the New COVID-19 Treatments Add-on Program (NCTAP).
Much, much more.
If the reader can help locate the above data, I would greatly appreciate it. Contact me at James.Roguski@gmail.com or call me directly at 310-619-3055.
VOLUNTEER HELP WANTED
At this point in my research into Veklury® (remdesivir) I realize that the world needs a lot more than I can do by myself, so I would like to clearly ask for volunteers to help in the following categories:
Help with the analysis of relational databases provided by the FDA Adverse Events Reporting System (FAERS) quarterly database. (See PDFs in the section above.)
Help with submitting FOIA requests for the missing data as well as additional communications that may need to be requested to support legal actions.
Legal help in filing a federal lawsuit requesting an injunction to stop the experimental use of Veklury® (remdesivir) on children under 12 years of age.
If you are willing to help, please contact me directly at 310-619-3055. James.Roguski@gmail.com
A federal court injunction stopping the use of Veklury® (remdesivir) in children under 12 years of age (including NEWBORN INFANTS) should be sought.
I am seeking legal assistance in filing a lawsuit in federal court seeking an injunction to stop the use of Veklury® (remdesivir) in children under 12 years of age.
Please contact me directly at 310-619-3055 if you wish to help.
VIOLATION #1:
Failure to perform adequate and well-controlled studies and analysis
This Emergency Use Authorization (EUA) violated the fundamental requirements for adequate and well controlled studies.
NO studies were referenced in the EUA that included children under 12 years of age. Instead, the FDA authorized the drug for use based on extrapolation of data from much older patients. (FACT #3)
Studies to determine safe and effective dosage where not conducted. Instead, “modeling and simulation” were used to determining dosage levels for children. (FACT #4)
Clear errors were made, resulting in recommended dosing levels being set at twice the amount received by an adult of average weight (176 pounds) on a pound-for-pound basis. (FACT #5)
These are not accepted analytical methods, as required by law.
21 CFR § 314.126 Adequate and well-controlled studies.
(b) An adequate and well-controlled study has the following characteristics:
(1) There is a clear statement of the objectives of the investigation and a summary of the proposed or actual methods of analysis in the protocol for the study and in the report of its results. In addition, the protocol should contain a description of the proposed methods of analysis, and the study report should contain a description of the methods of analysis ultimately used. If the protocol does not contain a description of the proposed methods of analysis, the study report should describe how the methods used were selected.
Also, properly controlled studies involving participants within the relevant age group were not conducted. As prescribed by law, the FDA was obligated to refuse to approve the application. The law requires that the FDA refuse to approve the drug application.
21 CFR § 314.125 Refusal to approve an NDA.
(b) FDA may refuse to approve an NDA for any of the following reasons, unless the requirement has been waived under § 314.90: [this has either not been done or not been publicly disclosed]
(3) The results of the tests show that the drug is unsafe for use under the conditions prescribed, recommended, or suggested in its proposed labeling or the results do not show that the drug product is safe for use under those conditions.
(4) There is insufficient information about the drug to determine whether the product is safe for use under the conditions prescribed, recommended, or suggested in its proposed labeling.
(5) There is a lack of substantial evidence consisting of adequate and well-controlled investigations, as defined in § 314.126, that the drug product will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in its proposed labeling.
(c) For drugs intended to treat life-threatening or severely-debilitating illnesses that are developed in accordance with §§ 312.80 through 312.88 of this chapter, the criteria contained in paragraphs (b) (3), (4), and (5) of this section shall be applied according to the considerations contained in § 312.84 of this chapter.
Please note the use of the word “shall” in (c) above, as it over-rides the use of the word “may” in (b) in regards to (3), (4) and (5) in the case of life-threatening illnesses.
VIOLATION #2:
Fraud in connection with emergency benefits.
Gilead Sciences and the FDA failed to consider numerous peer-reviewed published studies showing the lack of benefit of Veklury (remdesivir).
Gilead Sciences and the FDA also failed to consider numerous peer-reviewed published studies showing the potential that Veklury (remdesivir) could actually cause substantial and life-threatening harm.
Ignoring these material facts and hiding them from the general public may constitute fraud in connection with major disaster or emergency benefits.
The motive is financial gain.
18 USC § 1040 Fraud in connection with major disaster or emergency benefits
(a) Whoever, in a circumstance described in subsection (b) of this section, knowingly-
(1) falsifies, conceals, or covers up by any trick, scheme, or device any material fact; or
(2) makes any materially false, fictitious, or fraudulent statement or representation, or makes or uses any false writing or document knowing the same to contain any materially false, fictitious, or fraudulent statement or representation,
in any matter involving any benefit authorized, transported, transmitted, transferred, disbursed, or paid in connection with a major disaster declaration under section 401 of the Robert T. Stafford Disaster Relief and Emergency Assistance Act (42 U.S.C. 5170) or an emergency declaration under section 501 of the Robert T. Stafford Disaster Relief and Emergency Assistance Act (42 U.S.C. 5191), or in connection with any procurement of property or services related to any emergency or major disaster declaration as a prime contractor with the United States or as a subcontractor or supplier on a contract in which there is a prime contract with the United States, shall be fined under this title, imprisoned not more than 30 years, or both.
Opinion:
My personal opinion is that Veklury® (remdesivir) is at best ineffective, and at worst, it is a deadly chemical concoction that includes remdesivir and betadex sulfobutyl ether sodium (SBECD).
It is very clear that the federal government is financially incentivizing hospitals, health care facilities and now, outpatient facilities to preferentially use Veklury® (remdesivir) instead of any other therapy.
Every member of the government-hospital-medical-pharmaceutical-media industrial complex is under a moral, ethical and legal obligation to help expose the truth behind this ongoing experiment on American children.
I am advocating for the following:
Everyone who reads these words is morally and ethically obligated to share this information with everyone that they possibly can, especially with parents of children under 12 years of age.
Health care professionals need to look into their hearts and realize that they are responsible for their actions, regardless of what “people in positions of authority” direct them to do. They must stop administering EXPERIMENTAL Veklury® (remdesivir) to children under 12 years of age IMMEDIATELY.
Lawsuits should be filed seeking court orders to halt the use of Veklury® (remdesivir).
The EXPERIMENTAL use of Veklury® (remdesivir) in children under 12 years of age must STOP.
The Emergency Use Authorization that enables the use of Veklury® (remdesivir) in children under the age of 12 should be revoked.
All the data from all the trials ever done on Veklury® (remdesivir) must be released to the public.
All the data from all of the real world administration of Veklury® (remdesivir) to patients in hospitals, health care facilities and outpatient settings must be made publicly available.
Multiple criminal and/or grand jury investigations need to be started into the possibility that the authorization and approval of Veklury® (remdesivir) involved fraud, collusion and many other crimes against humanity, including millions of cases of child endangerment that have resulted in the unnecessary death of hundreds of thousands of Americans.
As evidence is revealed in the above mentioned investigations, charges must be filed and the perpetrators of these massive crimes against humanity must be brought to trial.
Call To Action:
Please help spread the word:
Several weeks ago I sent this information to hundreds of people who are active in the “COVID-19 truth community” and so far, disturbingly few people have responded.
Special thanks to the few people who have responded: Craig Jardula, David Knight, Ali Schultz, Janci C. Lindsay, Ph.D, Dr. Ryan Cole and Zoe Strickland from the World Council For Health.
I would greatly appreciate it if you, the reader, could share this information with everyone you possibly can.
Thanks for your precious time and attention.
I really appreciate it.
THIS SPACE RESERVED FOR FACT CHECK ARTICLES:
I always realize that I may be inaccurate in some way.
In this case, my God, I hope so.
Please check back here in the future.
If someone points out any factual errors in this article, I will CERTAINLY post corrections/retractions here.
If you make a good suggestion, post a cogent analysis or (even better) post EVIDENCE of criminal activity, I will certainly highlight it here:
by James Roguski
The old system is crumbling, and we must build its replacement quickly.
If you are fed up with the government, hospital, medical, pharmaceutical, media, industrial complex and would like to help build a holistic alternative to the WHO, then feel free to contact me directly anytime.
JamesRoguski.substack.com/about
310-619-3055
























































































READ THIS TOO... https://moderndiscontent.substack.com/p/remdesivir-covids-standard-of-care-942
THIS IS PART OF THE GLOBALIST PREDATOR WORLD ECONOMIC FORUM DESPOTS' EUGENICS/DEPOPULATION/GENOCIDE AGENDA - TOTAL SLAVERY FOR THE SURVIVORS. CRIMES AGAINST HUMANITY ON AN ATROCIOUS SCALE HITLER, MENGELE AND STALIN AND MAO COULD ONLY DREAM OF!