Evidence of Military Control
Operation Warp Speed was a merger of the Military Industrial Complex with the Pharmaceutical Hospital Emergency Industrial Complex designed to inflict biological warfare upon the world.
FOR COMPLETE DETAILS: NotSafeAndNotEffective.com
The response to COVID-19 was NOT a public health response.
It had nothing to do with science or medicine or public health.
It was and is a biological warfare program.
It was and continues to be democide.
The work done by Katherine Watt, Sasha Latypova and Debbie Lerman has been instrumental in exposing the true nature of the COVID-19 “response.”
KATHERINE WATT
Vaccination is legalized, decriminalized, government-sponsored, government-run torture, mutilation and homicide.
https://bailiwicknews.substack.com/p/vaccination-is-legalized-decriminalized
SASHA LATYPOVA
mRNA, Gene Therapies and Biomanufacturing Fraud
https://sashalatypova.substack.com/s/mrna-gene-therapies-and-biomanufacturing
My research summarized for a book chapter
https://sashalatypova.substack.com/p/my-research-summarized-for-a-book
DEBBIE LERMAN
Government’s National Security Arm Took Charge During the Covid Response
https://brownstone.org/articles/governments-national-security-arm-led-the-covid-response/
Vaccines Were Created by Military Contractors
https://rumble.com/v5sox5k-rfk-jr-says-vaccines-were-created-by-military-contractors.html
COVID-19 COVER-UP Goes Straight to the Top
https://www.youtube.com/watch?v=ERvURcpg3JE
The X/Twitter interview below was published on December 8, 2024
Transcript:
THE FATAL FLAW OF THE HEALTH FREEDOM MOVEMENT—it must admit the C19 op is planned genocide largely executed by the US military-industrial complex (1/9)
"We've uncovered the scheme of the crime...it has nothing to do with science or medicine or public health. It's...genocide."
Retired pharma R&D executive Sasha Latypova how much of the health freedom movement still refuses to admit that the COVID operation (my term) was premeditated genocide and not just a public health response rife with supposed mistakes. Latypova notes that while many of the members of the health freedom movement are willing to say COVID was a "mismanaged health event," they won't come out and espouse the truth: that the COVID operation was, in fact, a "crime" and a "genocide.”
My biggest frustration today, and over the years, is with all sides of [the health freedom movement] trying to position this whole situation as a scientific debate or medical issue or even a public health issue. The deluded side thinks it's a virus and it's a pandemic. The side that's ostensibly health freedom side says...'Oh, yeah, it's a pandemic, but [it's just a] mismanaged public health issue.'"
"[But] it's an intentional poisoning," Latypova notes, referencing the COVID injections.
"Even in 2021, I knew it was an intentional poisoning. [And] since then we've uncovered the whole scheme of the crime, the whole nature of the crime, and it has nothing to do with science or medicine or public health. It's a...genocide."
Latypova goes on to say that "if you're a truth-seeker...you have to move as the new data comes in; you have to make additional conclusions...[and yet]...the health freedom [movement]...will come to a certain point, but [go] no further." The pharma insider notes, "that's a hallmark of brand building to get political power versus seeking the truth....They [somehow] can't get past this point that this is a mismanaged health event."
Describing a key part of how the COVID genocide was committed, Latypova notes the following: "
We used to have a public health policy prior to 2018, 2019. We had a public health policy, which dealt with things like pandemics. Now, assume that this is a valid point—I personally believe pandemics do not exist, but even if you assume that this is a valid point and the government should have a policy for such situations... the policy said that Department of Health and Human Services is in charge of the policy, which is reasonable. And it also [called for] fairly reasonable public health measures, such as masking wasn't advised. There was... some quarantine measures or some advisory to stay home if you're sick, you know, some normal... public health advice. [But there were] no mandates of vaccines or anything like that.
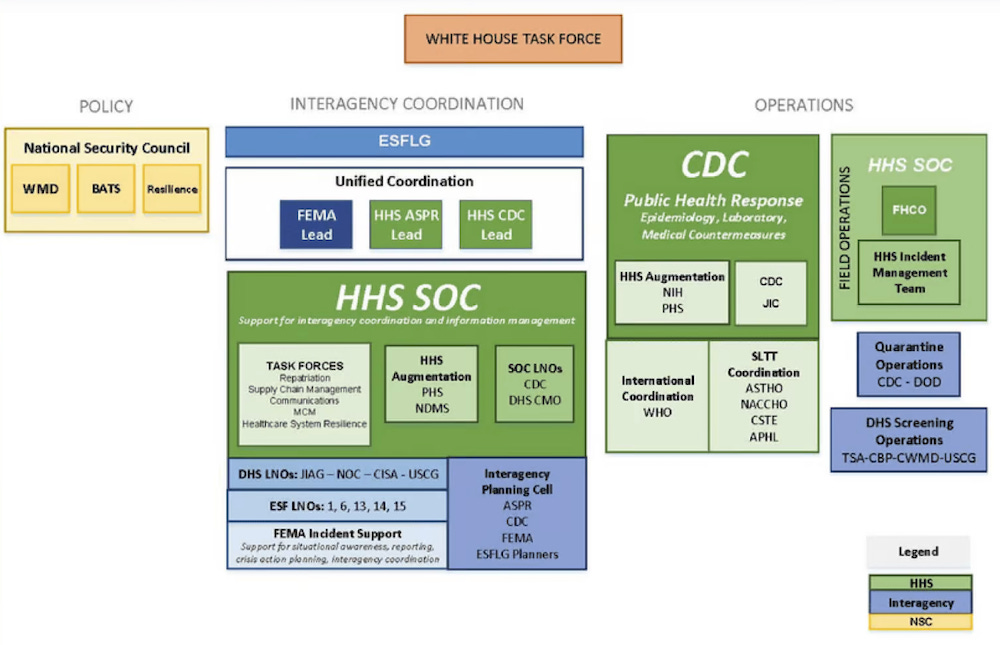
Now that policy was documented. [But] somehow in 2020, or maybe late 2019 or 2020, it's not clear when, [a] switch happened; that policy was replaced by a brand new policy....[one] that all of a sudden put the National Security Council in charge of pandemic policy. And that's a shocking switch. And still, only a few authors have commented on it.
Latypova goes on to say: "The first person who identified this was Debbie Lerman, to my knowledge, and she is a journalist writing [for the Brownstone Institute]. And so she wrote several articles about it. I found the documents...referenced. She and I also found additional documents after that. So, in charge of the whole policy is the National Security Council. [And the] National Security Council is an advisory body to the President of the United States, and consists of heads of military and intelligence; it does not have any representatives from Health and Human Services on the council...[And] this has been known now for at least two or three years.”
[Yet still] everybody is pinning [the COVID crimes] on Anthony Fauci. And I'm not defending him. He's a Mengele type, and he should be prosecuted for what he did both during the AIDS [crisis] and all the years that he's done similar crimes. But he wasn't in charge of this [COVID] policy. He [was at] NIH, NIAID, and, again, had nothing to do with the policy...He was part of designing certain things, and he was an advisor; HHS played an advisory role. So, obviously, he was involved in designing certain things, and he is clearly the spokesperson for the cabal. But it's not his policy.
It's the [policy of the] National Security Council [and U.S.] military and intelligence."
Sasha Latypova Exposes The Military For Covid-19 Countermeasures - Evidence of Intent to Harm
Katherine Watt and Sasha Latypova Expose Why The Jab is a Bioweapon Military Product, Not a Vaccine
A Department of Defense DEMONstration
Evidence of the Conspiracy to Commit Mass Murder by the U.S. DOD, HHS, Pharma Cartel.
https://MedicalCountermeasures.gov
Robert F. Kennedy Jr. and Sasha Latypova Discuss Militarized Healthcare and Corruption in the Covid Vaccine Manufacturing
https://rumble.com/v5cxma5-robert-f-kennedy-jr-sasha-latypova.html
Public Health Worldwide Has Been Militarized Creating "Kill Zones" For Global Depopulation and Control
The US military is actively engaged in an organized criminal enterprise to injure and kill large numbers of military personnel and civilians without detection or legal impediment.
One of the most useful tools in the arsenal — because it strikes an effective balance between the killers’ two primary interests in speed and deniability — is the deployment of prohibited biochemical weapons labeled as FDA-authorized or FDA-approved ‘vaccines.’
The ‘vaccine’-based killing program is an extension of medical and psychological torture and homicide programs conducted to kill millions of people (disabled, mentally-ill, Jewish, Catholic, Protestant, Roma, politically-dissident and many more), especially during and since World War II, including but not limited to Aktion T-4 and the Soviet gulag system.
The most recent and most visible phase of the program launched in the US in early 2020, under the title Operation Warp Speed, and resulted in global deployment of psychological fraud and control programs including terrorizing propaganda; social isolation; mask mandates; diagnostic tests; manipulated data presentations (i.e. “dashboards”); prohibition on treatments for symptoms; and financial coercion of hospitals and nursing home death protocols (sedatives, ventilators and toxins).
These components were followed by distribution of three brands of biochemical weapons (Pfizer-BioNTech, Moderna and Johnson & Johnson) with an unknown number of different batch formulations.
The biochemical weapons were and are developed and manufactured under redacted contracts, to DoD specifications, non-compliant with FDA pharmaceutical manufacturing regulations.
They are delivered — by way of the Strategic National Stockpile and DoD transport systems, non-compliant with FDA pharmaceutical distribution regulations — to retail pharmacies, nursing homes, hospitals, clinics, workplaces, schools, parking lots and medical offices, and from there into the hands of pharmacists, nurses and other ‘vaccinators,’ for injection into military targets at community-level ‘vaccination’ clinics (“points of dispensing”/PODs).
To date, the contents have not been publicly disclosed.
Independent researchers have identified some but not all components of some vials diverted from the Strategic National Stockpile supply chain, including heavy metals, genetic code fragments, and many other contaminants not listed on applications submitted to regulators by manufacturers, who are working under redacted contracts for the US Department of Defense.
These biochemical weapons are exempt from, and therefore non-compliant with, all pharmaceutical regulation.
As such, DoD, CDC and FDA took great care to not produce any pharmaceutical chain-of-custody paper trail between suppliers, manufacturers, distributors, ‘vaccinators’ and targets.
If they can produce any chain of custody records at all, those records will demonstrate that the products are military-grade biological and chemical weapons passed through the Strategic National Stockpile — not handled by regulated pharmaceutical distributors — under direct military control from the point at which raw materials entered production facilities to delivery of finished vials to retail pharmacies, medical offices, drive-through vaccination centers and other “points of dispensing.”
https://bailiwicknews.substack.com/p/usa-v-dr-kirk-moore-et-al
I believe all of the parties to the contracts — including but not limited to the Pfizer signatories — have been and continue to jointly, collaboratively, cooperatively, intentionally and maliciously commit fraud, mass murder, and war crimes. They have been and continue to commit those crimes against non-parties to the contracts…”
These biochemical weapons are exempt from, and therefore non-compliant with, all pharmaceutical regulation. As such, DoD, CDC and FDA took great care to not produce any pharmaceutical chain-of-custody paper trail between suppliers, manufacturers, distributors, ‘vaccinators’ and targets.
If they can produce any chain of custody records at all, those records will demonstrate that the products are military-grade biological and chemical weapons passed through the Strategic National Stockpile — not handled by regulated pharmaceutical distributors — under direct military control from the point at which raw materials entered production facilities to delivery of finished vials to retail pharmacies, medical offices, drive-through vaccination centers and other ‘points of dispensing’…”
https://bailiwicknews.substack.com/p/on-contracts-consortium-agreement
On the significance of 21 USC 360bbb-3(k): "use" of EUA products "shall not constitute clinical investigation."
The primary purpose of all the statutory, regulatory changes and guidance document revisions year after year, page after page, is to keep people from, first, understanding the war crimes as war crimes, and — if people do figure it out — keep them chasing their tails trying to find the FDA loophole that the war criminals somehow failed to close, through which somebody might someday be able to get them to stop killing us.
In the meantime, they just keep killing, and we don’t find loopholes, because the complexity of the web is impenetrable, and the program is not an FDA-regulated medical treatment program anyway: it’s a military-operated global genocide.
https://bailiwicknews.substack.com/p/on-the-significance-of-21-usc-360bbb
Whatever is in the biochemical weapons bearing Pfizer and other pharma labels, is there because US SecDefs and their WHO-BIS handlers ordered it to be there.
They are trying to shield the mRNA technology and vaccination program platforms, and the public health emergency geopolitical and legal platforms from growing public understanding of what’s really going on, so that the Monster can keep using public health emergency laws, orchestrated pandemics, vaccines, and mRNA-platform poisons to sicken and kill many more people for many years to come.
https://bailiwicknews.substack.com/p/whatever-is-in-the-biochemical-weapons
The response to COVID-19 was clearly NOT determined by the Department of Health and Human Services (HHS), as admitted by the current and former Secretaries. Watch the two brief clips below:
Secretary of HHS (Under Biden) Xavier Becerra (June 22, 2023)
https://www.youtube.com/watch?v=nCsPVXp-4EA&t=2370s
Former Secretary of HHS (under Trump) Alex Azar (June 22, 2023)
https://www.youtube.com/watch?v=nCsPVXp-4EA&t=2099s
Dr. Robert Malone
COVID jabs were a CIA operation to depopulate the world
Debbie Lerman
Debbie Lerman was the first to expose the fact that the National Security Council was in charge of the response to COVID-19, NOT the Department of Health and Human Services.
Debbie Lerman’s articles are below:
November 3, 2022
Government’s National Security Arm Took Charge During the Covid Response
https://brownstone.org/articles/governments-national-security-arm-led-the-covid-response/
November 7, 2022
Was there a Covid Response Plan? If So, Where Is It?
https://brownstone.org/articles/was-there-a-covid-response-plan-if-so-where-is-it/
January 18, 2023
Lockdowns Were Counterterrorism, Not Public Health
https://brownstone.org/articles/lockdowns-counterterrorism-not-public-health/
February 17, 2023
Vaccine Harms Are Biodefense Plan’s Collateral Damage
https://brownstone.org/articles/vaccine-harms-are-biodefense-plans-collateral-damage/
June 26, 2023
Pandemic Leaders Were Biodefense Puppets and Profiteers
https://brownstone.org/articles/pandemic-leaders-were-biodefense-puppets-and-profiteers/
December 6, 2023
Covid mRNA Vaccines Required No Safety Oversight
https://brownstone.org/articles/covid-mrna-vaccines-required-no-safety-oversight/
January 14, 2024
Covid mRNA Vaccines Required No Safety Oversight: Part Two
https://brownstone.org/articles/covid-mrna-vaccines-required-no-safety-oversight-part-two/
January 21, 2024
Why Was the BioNTech/Pfizer mRNA Vaccine Not Recalled in February 2021?
February 9, 2024
DOD Told Pharma Exec the Virus “Posed a National Security Threat” on Feb. 4, 2020
May 13, 2024
Legal Context Behind the Bioweapon Hypothesis
https://brownstone.org/articles/legal-context-behind-the-bioweapon-hypothesis/
September 29, 2020
How Operation Warp Speed's Big Vaccine Contracts Could Stay Secret
Operation Warp Speed is issuing billions of dollars' worth of coronavirus vaccine contracts to companies through a nongovernment intermediary, bypassing the regulatory oversight and transparency of traditional federal contracting mechanisms, NPR has learned.
Instead of entering into contracts directly with vaccine makers, more than $6 billion in Operation Warp Speed funding has been routed through a defense contract management firm called Advanced Technologies International, Inc. ATI then awarded contracts to companies working on COVID-19 vaccines.
October 6, 2020
Operation Warp Speed is Using a CIA-Linked Contractor to Keep Covid-19 Vaccine Contracts Secret
On September 21st, HHS Secretary Alex Azar told FOX Business that all Operation Warp Speed vaccine manufacturers would be exempt from liability for any damages their vaccines may cause and that those who administer those vaccines would also not be liable for damages.
“Under the PREP Act, which is a provision in Congress, any treatment or vaccine for purposes of a national emergency pandemic like this actually comes with liability protection. Both the product as well as those who administer it or provide it,” Azar stated during the televised interview.
The PREP Act that Azar referenced was originally signed into law in 2005 but was updated this past April, a few weeks before Operation Warp Speed was announced, so that vaccine and therapeutic manufacturers “cannot be sued for money damages in court” over injuries caused by medical countermeasures for Covid-19.
https://unlimitedhangout.com/2020/10/investigative-reports/operation-warp-speed/
Pfizer/U.S. Government Contract:
The Government will provide Pfizer with no less than thirty (30) days' written notice prior to releasing, in response to a Freedom of Information Act (FOIA) request, any document submitted by Pfizer to Government. During this 30-day period, Pfizer shall have the right to notify Government which documents, if any, contain trade secrets of Pfizer, BioNTech or their respective collaboration partners (or other information legally withholdable from release under FOIA). (page 20)
https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf
CLICK HERE, HERE AND HERE for links to government contracts.
Below are links to copies of the contracts entered into by the US government for technologies to combat the COVID-19 pandemic. KEI is updating this page as we gain access to more contracts, and less redacted versions. Agreements related to the Medical CBRN Defense Consortium (MCDC) whose agreements are executed by Advanced Technology International (ATI) are noted with “(via ATI)”.
KEI has also created a US COVID-19 contract spreadsheet to track metadata of the agreements, as well as to compare the terms contained therein for intellectual property rights, data, and other topics. (Note: At this time, this spreadsheet is being continually updated.)
KEI maintains a separate page of US government contracts concerning needle and syringe production related to the COVID-19 response.
The list below contains COVID-19-related contracts obtained via Freedom of Information Act requests, Securities & Exchange Commission filings, and HHS reading room files. The bulk of KEI’s database of contracts, however, was obtained via FOIA requests and lawsuits filed by KEI.
A KEI review of contracts between the United States government and private companies to provide countermeasures for COVID 19 illustrates the simplicity of the legal mechanism used by the federal government to eliminate exclusive rights to use patented inventions for federal programs, and the surprising frequency that this was done for COVID 19 countermeasures.
https://www.keionline.org/37987
https://www.keionline.org/wp-content/uploads/KEI-bn-2022-1.pdf
COVID-19 Contracts
Below is a comprehensive list of all US government COVID-19-related contracts obtained by KEI via FOIA.
Advanced Technology International (ATI) – Underlying contract to execute MCDC and COVID-19 contracts on behalf of the federal government.
DoD-ATI Other Transaction Authority Agreement W15QKN1691002-P00085. April 8, 2016.
DoD-ATI Other Transaction Authority Agreement W15QKN1691002-P00085. April 8, 2016. (Version obtained November 30, 2020 from HHS FOIA Reading Room)
Aerpio – respiratory condition treatment.
DOD-Aerpio Project Approval Letter W81XWH1590001. July 28, 2020.
Altimmune – therapeutic.
DoD-Altimmune Project Approval Letter W81XWH159001. June 17, 2020.
DoD-Altimmune Revised Project Approval Letter (3) W81XWH159001. February 3, 2021.
DoD-Altimmune Revised Project Approval Letter (2) W81XWH159001. December 15, 2020.
America’s Blood Center – convalescent plasma.
HHS/ASPR/BARDA-America’s Blood Center Contract 75A50120000094 (includes Mods 1-8). April 17, 2020.
DOD-America’s Blood Centers Contract W911QY2190006. October 30, 2020.
ANP Technologies – diagnostics.
DoD-ANP Technologies Contract W911QY20D0019 (includes Mods 1-3). May 29, 2020.
DOD-ANP Technologies Supply Order W911QY20D0019 (includes Mods 1-3). June 2, 2020.
DOD-ANP Technologies Supply Order W911QY20P0141 (includes Mod 1). April 17, 2020.
AstraZeneca – vaccine.
HHS/ASPR/BARDA-AstraZeneca Advanced Agreement to Other Transaction Authority Agreement 75A501-20-C-00114. May 20, 2020.
HHS/ASPR/BARDA-AstraZeneca Modification of OTA Agreement 75A501-20-C-00114 MODP00001. July 31, 2020.
AstraZeneca – vaccine.
DoD-AstraZeneca Other Transaction Authority Agreement W15QKN2191003. October 28, 2020.
AstraZeneca – prophylactic monoclonal antibody.
DOD-AstraZeneca Contract W911QY2190001 (includes Mods 1, 2, 3, and 5). October 9, 2020.
AstraZeneca – therapeutic.
DoD-AstraZeneca Contract W911QY20C0119 (includes Mod 1). September 30, 2020.
DoD-AstraZeneca Contract W911QY20C0119 (includes Mod 1). September 30, 2020. (Version obtained by FOIA)
Atlantic Diving Supply – no-contact thermometers.
DOD-Atlantic Diving Supply Contract W911QY18D0019. September 16, 2020.
Beckman Coulter – diagnostic-related.
HHS/ASPR/BARDA-Beckman Coulter Contract 75A50119C00078. September 30, 2019.
HHS/ASPR/BARDA-Beckman Coulter Contract 75A50119C00078-P00001. May 15, 2020.
HHS/ASPR/BARDA-Beckman Coulter Contract 75A50120C00189. September 28, 2020.
Biofire Defense – diagnostics.
DoD-Biofire Defense Supply Order W911QY13D0080 Contract W911QY20F0271. April 24, 2020.
DoD-Biofire Defense Supply Order W911QY13D0080 Contract W911QY20F0171 (includes Mods 1-2). May 23, 2020.
DOD-Biofire Defense Supply Order W911QY20F0196 and W911QY20F0165 Contract W911QY13D0080 (includes Mod 1 of W911QY20F0196). April 17, 2020.
BCG Federal Corp – COVID-19-related support services.
DoD-BCG Federal Corp Contract W911QY20P0198. June 28, 2020.
Centivax – therapeutic.
DOD-Centivax Project Approval Letter W81XWH1590001. August 19, 2020.
Cepheid – test kits.
DoD-Cepheid Contract W911QY20C0046 (includes Mods 1, 2, 3, 5, and 6). April 5, 2020.
DoD-Cepheid Contract W911QY20P0154 (includes Mods 1-6). April 27, 2020.
DoD-Cepheid Contract W911QY20P0195 (includes Mod 2). June 12, 2020.
DoD-Cepheid Technical Direction Letter W15QKN1691002. September 14, 2020.
DOD-Cepheid Contract W911QY20C0124 (includes Mods 1-5, Supply Order W911QY21F0116 (January 22, 2021) + 1 Mod, and Supply Order W911QY21F0205 (March 25, 2021)). September 24, 2020.
ChemBio Diagnostic – antigen test.
HHS/ASPR/BARDA-ChemBio Diagnostic Contract 75A50120C00138. July 3, 2020.
HHS/ASPR/BARDA-ChemBio Diagnostic Contract 75A50120C00138-P00001. July 20, 2020.
Controlant – services.
DoD-Controlant Contract W911QX21C0010 (includes Mods 1-4). November 25, 2020.
DoD-Controlant Contract W911QX21C0010 Mods 5, 7, and 8. May 10, 2021.
Corning Incorporated – vaccine manufacturing supplies.
DoD-Corning Contract W911NF2030004. June 5, 2020.
Cue Health – diagnostics manufacturing supplies.
DoD-Cue Health Other Transaction Authority Agreement W911NF2190001. October 13, 2020.
Cue Health – diagnostics.
HHS-Cue Health Contract HHSO100201800016C. June 4, 2018.
HHS-Cue Health Contract HHSO100201800016C-P00001. September 1, 2019.
HHS-Cue Health Contract HHSO100201800016C-P00002. March 21, 2020.
HHS-Cue Health Contract HHSO100201800016C-P00003. May 13, 2020.
HHS-Cue Health Contract HHSO100201800016C-P00004. September 22, 2020.
Culmen – PPE.
DOD-Culmen International Contract W911QY18D0042 (includes Mods 1-8). April 22, 2020.
Cytiva (Global Life Sciences Solutions) – vaccine manufacturing supplies.
DoD-Cytiva Contract W911NF2130001 (includes Mod 1). October 13, 2020.
Cytovale – COVID-related sepsis assessment.
HHS-Cytovale Contract 75A50119C00072. September 28, 2019.
HHS-Cytovale Contract 75A50119C00072-P00001. April 7, 2020.
Diasorin – assays.
HHS/ASPR/BARDA-DiaSorin Contract 75A50120C00017. March 11, 2020.
Diomics Corporation – diagnostics.
DoD-Diomics Corporation Project Approval Letter W81XWH159001. August 17, 2020.
DoD-Diomics Corporation Revised Project Approval Letter W81XWH159001. February 2, 2021.
Eli Lilly – therapeutic.
DoD-Eli Lilly Contract W911QY21C0016 (includes Mods 1-5). October 27, 2020.
DOD-Eli Lilly Contract W911QY21D0012-P00002. April 7, 2021.
DOD-Eli Lilly Contract W911QY21F0167-P00003. May 27, 2021.
Emergent Biosolutions – convalescent plasma manufacturing and clinical studies.
DOD-Emergent Biosolutions Canada Contract W911QY2090013 (includes Mod 1). June 24, 2020.
Emergent Biosolutions – vaccine manufacturing.
HHS/ASPR/BARDA-Emergent Biosolutions Contract HHSO100201200004I. June 15, 2012. (Version obtained via SEC filing)
HHS/ASPR/BARDA-Emergent Biosolutions Contract HHSO100201200004I -Task Order 75A50120F33007. May 24, 2020. (Version obtained via SEC filing)
HHS/ASPR/BARDA-Emergent Biosolutions Contract HHSO100201200004I -Task Order 75A50120F33007. May 24, 2020. (Version obtained via FOIA)
HHS/ASPR/BARDA-Emergent Biosolutions Contract HHSO100201200004I-75A50120F33007-P00001. August 24, 2020.
HHS/ASPR/BARDA-Emergent Biosolutions Contract HHSO100201200004I-75A50120F33007-P00002. May 13, 2020.
HHS/ASPR/BARDA-Emergent Biosolutions Contract HHSO100201200004I-75A50120F33007-P00003. October 7, 2020.
Emory University – therapeutic.
DoD (via ATI)–Emory University Technical Direction Letter Other Transaction Authority W15QKN1691002. July 1, 2020.
Empatica – diagnostics technology.
HHS/ASPR/BARDA-Empatica Contract 75A50120C00132. June 18, 2020.
Empatica – diagnostics technology.
DoD-Empatica Project Approval Letter W81XWH159001. September 10, 2020.
Evidation Health – COVID-19 detection and forecasting model.
HHS/ASPR/BARDA-Evidation Health Contract 75A50120C00091. April 17, 2020.
Federal Resources Supply – collection kits.
DOD-Federal Resources Supply Contract W911QY18D0060 – Order W911QY20F0215 (includes Mods 1-2). April 24, 2020.
DOD-Federal Resources Supply Contract W911QY18D0060 – Order W911QY20F0226 (includes Mods 1-3). May 4, 2020.
DOD-Federal Resources Supply Contract W911QY18D0060 – Order W911SR20F0039 (includes Mods 1-5). April 22, 2020.
Fitbit – diagnostics technology.
DoD-Fitbit Project Approval Letter W81XWH1590001. September 23, 2020.
Flir – thermometers.
DOD-Flir Commercial Contract W911QY20P0149. April 22, 2020.
Fujifilm – therapeutic.
DoD (via ATI)–Fujifilm Project Approval Letter 2009COVID19006 under Other Transaction Authority Agreement W81XWH1590001. July 9, 2020.
DoD (via ATI)–Fujifilm Revised Project Approval Letter 2009COVID19006 under Other Transaction Authority Agreement W81XWH1590001. December 14, 2020.
DoD (via ATI)–Fujifilm Statement of Work 2009COVID19006 under Other Transaction Authority Agreement W81XWH1590001. December 14, 2020.
Genentech (Roche) -therapeutic.
HHS/ASPR/BARDA-Genentech Other Transaction Authority Agreement HHSO100201800036C. September 27, 2018.
HHS/ASPR/BARDA-Genentech Modification of OTA Agreement HHSO100201800036C. March 27, 2020.
GenMark Diagnostics – diagnostic.
HHS/ASPR/BARDA-GenMark Diagnostics Contract 75A50120C00022. March 20, 2020.
HHS/ASPR/BARDA-GenMark Diagnostics Contract 75A50120C00022-P00001. May 28, 2020.
HHS/ASPR/BARDA-GenMark Diagnostics Contract 75A50120C00022-P00002. July 20, 2020.
GlaxoSmithKline – vaccines.
DoD/ASPR/BARDA-GlaxoSmithKline Contract W15QKN20C0048. July 30, 2020.
DoD/ASPR/BARDA-GlaxoSmithKline Contract W15QKN20C0048-P00001. July 30, 2020.
DoD/ASPR/BARDA-GlaxoSmithKline Contract W15QKN20C0048-P00002. September 16, 2020
Global Life Sciences – vaccine supply manufacturing.
DOD-Global Life Sciences Solution Contract W911NF2130001 P00003. July 29, 2021.
Golden Max – infusion pump kits.
DoD-Golden Max Contract W81XWH20D0057 (includes Mod 1). April 17, 2020.
Grifols Therapeutics – convalescent plasma.
DoD (via ATI)-Grifols Therapeutics Technical Direction Letter Other Transaction Authority Agreement W15QKN1691002. September 1, 2020.
Grand River Aseptic Manufacturing (GRAM) – vaccine and therapeutic manufacturing.
DoD-Grand River Aseptic Manufacturing (GRAM) Contract W3110Y2OCC086-P00003. August 4, 2020.
DoD-Grand River Aseptic Manufacturing (GRAM) Contract W3110Y2OCC086 – Mods 3 and 1-5. August 4, 2020. (Version obtained via FOIA)
DoD-Grand River Aseptic Manufacturing (GRAM) Contract W3110Y2OCC086 Mods 1-5. September 1, 2020.
Hologic – testing assays.
HHS/ASPR/BARDA-Hologic Contract 75A50120P00069. April 29, 2020.
HHS/ASPR/BARDA-Hologic Contract 75A50120P00100. August 7, 2020.
HHS/ASPR/BARDA-Hologic Contract 75A50120P00100-P00001. October 29, 2020.
Icon – clinical trials.
DoD-Icon Other Transaction Authority Agreement W911QY2090007 (includes Mods 1 and 4). April 27, 2020.
IIT Research Institute – tech transfer for virus neutralization assay.
DOD-IIT Research Institute Contract W911QY21C0014. November 30, 2020.
Immunome – antibody-related (redactions).
DOD-Immunome Contract W911QY2090019. July 3, 2020.
Inbios International – diagnostics.
DoD-Inbios International Contract W81XWH20F0253 (includes Mod 1). June 15, 2020.
Inbios – antibody diagnostic.
HHS-InBios Contract 75A50120C00090. April 26, 2020.
Inhalon Biopharma – inhaled prevention/therapeutic.
DoD-Inhalon Biopharma Project Approval Letter W81XWH1590001. September 11, 2020.
Inovio Pharmaceuticals – vaccine delivery.
DoD-Inovio Pharmaceuticals Contract W911QY20C0084. June 18, 2020. (Version obtained from SEC filings)
DoD-Inovio Pharmaceuticals Contract W911QY20C0084. June 18, 2020. (Version obtained via FOIA request)
DoD-Inovio Pharmaceuticals Other Transaction Authority Agreement W911QY2090016. June 22, 2020. (Version obtained from SEC filings)
DOD-Inovio Pharmaceutical Other Transaction Authority Agreement W911QY2090016. June 22, 2020. (Version obtained via FOIA request)
Johns Hopkins University – clinical trials of convalescent plasma.
Johnson&Johnson (Janssen) – vaccine.
HHS/ASPR/BARDA-Janssen Other Transaction Authority Agreement HHSO100201700018C. August 15, 2017. (Version obtained by FOIA March 31, 2021).
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendment 6. February 11, 2020.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendment 6. February 11, 2020. (Version obtained by FOIA March 31, 2021).
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendment 7. March 20, 2020.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendment 8. March 27, 2020.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendment 8. February 11, 2020. (Version obtained by FOIA March 31, 2021).
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendments 1, 2, 3 (dates redacted) 4 (date not listed), 5 (December 19, 2019), 9 (date not listed), 10 (August 21, 2020). (Version obtained by FOIA June 2021)
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201700018C-Amendment 6. February 11, 2020. (Version obtained by FOIA June 2021)
Johnson&Johnson (Janssen) – therapeutic.
HHS/ASPR/BARDA-Janssen Other Transaction Authority Agreement HHSO100201800012C. September 21, 2018.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201800012C-Amendment 1. April 4, 2019.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201800012C-Amendment 2. August 1, 2019.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201800012C-Amendment 3. January 31, 2020.
HHS/ASPR/BARDA-Janssen Amendment to OTA Agreement HHSO100201800012C-Amendment 4. February 14, 2020.
HHS/ASPR/BARDA-Janssen Other Transaction Authority Agreement HHSO100201800012C. September 21, 2018. (Version obtained via FOIA June 2021)
Johnson&Johnson (Janssen) – vaccine.
DoD (via ATI)-Janssen Other Transaction Authority Agreement W15QKN1691002-P00081. August 5, 2020.
DoD (via ATI)-Janssen Other Transaction Authority Agreement W15QKN1691002-P00081-Mod00001. September 21, 2020. (Version obtained by FOIA)
Just Evotec – therapeutic manufacturing.
DOD-Just Evotec Contract W911QY2090015 (includes Mods 1-3). July 7, 2020.
Kalman – labor support services.
DOD-Kalman W911QY19F0147-P00042. March 1, 2021.
KPMG – prototype process for mAb infusion capacity.
DOD-KPMG W912CG2190001-P00001. June 16, 2021.
Lumen Bioscience – therapeutic.
DoD-Lumen Bioscience Project Approval Letter W81XWH1590001. July 24, 2020.
DoD-Lumen Bioscience Revised Project Approval Letter (2) W81XWH1590001. August 11, 2020.
DoD-Lumen Bioscience Revised Project Approval Letter (3) W81XWH1590001. December 9, 2020.
DoD-Lumen Bioscience Revised Project Approval Letter (4) W81XWH1590001. February 4, 2021.
Luminex – antibody assay.
HHS/ASPR/BARDA-Luminex Contract 75A50120C00179. September 23, 2020.
HHS/ASPR/BARDA-Luminex Contract 75A5012C00037. March 26, 2020.
HHS/ASPR/BARDA-Luminex Contract 75A50120C00043. March 27, 2020.
Maxim – diagnostics.
DoD-Maxim Contract W911QY20D0018 (includes Mods 1-4). May 11, 2020.
MBio – antibody diagnostic.
HHS-MBio Diagnostics Contract 75A50120C00130. June 17, 2020.
Merck Sharp & Dohme – vaccine.
(Note: HHSO100201600031C began as a contract to Bioprotection Systems for an Ebola vaccine, Merck entered into a collaboration agreement with BPS in 2014)
HHS/ASPR/BARDA–Bioprotection Systems Corporation Contract HHSO100201600031C. September 29, 2016.
HHS/ASPR/BARDA–Bioprotection Systems Corporation Contract HHSO100201600031C-Attachment 2-Milestones. May 19, 2017.
HHS/ASPR/BARDA–Bioprotection Systems Corporation Contract HHSO100201600031C-Attachment 1-Statement of Work. May 19, 2017.
HHS/ASPR/BARDA–Bioprotection Systems Corporation Contract HHSO100201600031C-P00001. April 27, 2017.
HHS/ASPR/BARDA–Bioprotection Systems Corporation Contract HHSO100201600031C-P00002. September 6, 2017.
HHS/ASPR/BARDA–Bioprotection Systems Corporation Contract HHSO100201600031C-P00003. May 23, 2018.
HHS/ASPR/BARDA-Merck Sharp & Dohme Contract Amendment HHSO100201600031C-P00004. May 9, 2019.
HHS/ASPR/BARDA-Merck Sharp & Dohme Contract Amendment HHSO100201600031C-P00005. April 14, 2020. (Version obtained March 2021 via FOIA lawsuit)
HHS/ASPR/BARDA-Merck Sharp & Dohme Contract Amendment HHSO100201600031C-P00005. April 14, 2020.
Merck Sharp & Dohme – therapeutic.
DoD-Merck Sharp & Dohme Other Transaction Authority Agreement W911QY2190001. December 2, 2020.
Merck Sharp & Dohme – therapeutic.
DOD-Merck Sharp & Dohme Contract W911QY21C0031. June 7, 2021.
Microbiologics – tech transfer for virus neutralization assay.
DOD-Microbiologics Contract W911QY21C0012 (includes Mod 1). November 9, 2020.
Minnesota Medical Warehouse – hand sanitizer.
DOD-Minnesota Medical Warehouse Contract W911SR20P0010 (includes Mods 1-3). April 30, 2020.
Moderna – vaccine.
HHS/ASPR/BARDA-Moderna Contract 75A50120C00034. April 16, 2020.
HHS/ASPR/BARDA-Moderna Contract 75A50120C00034. April 16, 2020. (Less redacted version obtained via FOIA lawsuit on February 8, 2021)
HHS/ASPR/BARDA-Moderna Contract 75A50120C00034-P00003. July 25, 2020.
Moderna – vaccine manufacturing.
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100. August 9, 2020. (Version obtained from Moderna SEC filings)
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100. August 9, 2020. (Version obtained October 29, 2020 from HHS FOIA Reading Room)
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100. August 9, 2020. (Version obtained November 9, 2020 from HHS FOIA Reading Room)
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100-P00001. August 9, 2020.
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100-P00002. September 11, 2020.
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100-P00003. December 11, 2020.
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100-P00004. February 11, 2021. (Obtained via FOIA)
DoD/ASPR/BARDA-Moderna Contract W911QY20C0100 – Mods 7-10. June 15, 2021.
MRI Global – mobile medical laboratories.
DOD-MRI Global Contract W911SR20F0054 – Mods 1-2. May 27, 2020.
Murtech – diagnostics.
DoD-Murtech Contract W911QY20D0017 (includes Mods 1-2). May 11, 2020.
Nanomix – antibody diagnostics.
HHS/ASPR/BARDA-Nanomix Contract 75A50120C00060. April 2, 2020.
HHS/ASPR/BARDA-Nanomix Contract 75A50120C00060-P00001. June 19, 2020.
HHS/ASPR/BARDA-Nanomix Contract 75A50120C00060-P00002. July 24, 2020.
New Horizons Diagnostics Corp. – diagnostic.
Novavax – vaccine.
DoD-Novavax Contract W911QY20C0077 (includes Mod 1). June 4, 2020.
DoD-Novavax Contract W911QY20C0077 – Mods 1-4. June 4, 2020.
Novavax – vaccine.
DoD (via ATI)-Novavax Other Transaction Authority Agreement W15QKN1691002. June 25, 2020. (Version obtained via SEC filing)
DoD (via ATI)-Novavax Technical Direction Letter W15QKN1691002. July 6, 2020. (Version obtained November 30, 2020 from HHS FOIA Reading Room)
DoD (via ATI)–Novavax Statement of Work W15QKN1691002. (Version obtained via FOIA lawsuit)
DoD (via ATI)–Novavax Technical Direction Letters and Statements of Work W15QKN1691002. July 6, 2020 and December 21, 2020. (Version obtained via FOIA lawsuit)
NOWDiagnostics – diagnostics.
HHS/ASPR/BARDA-NOWDiagnostics Contract 75A50120C00156. August 27, 2020.
Ology Bioservices (previously called Nanotherapeutics, Inc.) – vaccine manufacturing.
DoD-Ology Bioservices Other Transaction Authority Agreement-W911QY2090003 Contract and Mods 1-5,8-10,12,14,15,17,23-25 . February 21, 2020
DoD-Ology Bioservices Other Transaction Authority Agreement OTA-W911QY2090003. February 21, 2020.
DoD-Ology Bioservices Other Transaction Authority Agreement Appendix OTA-W911QY2090003-Appendix-A-4. March 20, 2020.
DoD-Ology Bioservices Contract W911QY2090003-MOD-P00005. March 22, 2020.
Ology Bioservices (previously called Nanotherapeutics, Inc.) – vaccine manufacturing.
DoD-Ology Bioservices Contract W911QY20C0101 (includes Mod 1). August 17, 2020.
Ophirex – therapeutic.
DoD-Ophirex Contract W81XWH20C0066 (includes Mods 1-3). June 19, 2020.
Orasure – rapid antigen test.
HHS/ASPR/BARDA-Orasure Contract 75A50120C00061. Date not listed.
HHS/ASPR/BARDA-Orasure Contract 75A50120C00122. May 27, 2020.
Ortho Clinical – antibody assays.
HHS/ASPR/BARDA-Ortho Clinical Contract 75A50120C00123. June 12, 2020.
HHS/ASPR/BARDA-Ortho Clinical Contract 75A50120C00123-P00001. August 28, 2020.
HHS/ASPR/BARDA-Ortho Clinical Contract 75A50120P00103. September 18, 2020.
Oxford Nanopore Technologies – DNA sequencing.
DoD-Oxford Nanopore Technologies Contract W911QX20P0073 (includes Mod 1). April 22, 2020.
Partner Therapeutics, Inc. – therapeutic.
DOD (via ATI)-Partner Therapeutics Technical Direction Letter OTA W15QKN1691002. August 13, 2020.
Patricio Enterprises – COVID-19 mobile lab staffing.
DoD-Patricio Enterprises Supply Order W911QY2OF0270-P00001 Contract W911QY18D0251. June 3, 2020.
DOD-Patricio Enterprises Contract W911QY19F0133-P00037. December 7, 2020.
Pfizer – vaccine.
DoD (via ATI)-Pfizer Technical Direction Letter OTA W15QKN1691002. July 21, 2020.
Pfizer – vaccines.
DoD-Pfizer Contract W15QKN21C0012 (includes Mod 310). December 22, 2020.
Pfizer – manufacturing.
DVA-Pfizer Contract V797D40269-P00376. June 1, 2021.
Pfizer – vaccine donation.
DoD-Pfizer Contract W58P0521C0002. July 30, 2021.
DOD-Pfizer Contract W58P0521C0002 Contract and P00376. July 30, 2021.
Pharm Olam – therapeutic clinical trial.
DOD-Pharm Olam Contract W911QY2190014. December 31, 2020.
Philips North America – diagnostics technology.
HHS/ASPR/BARDA-Philips North America Contract 75A50120C00097. May 7, 2020.
DoD-Philips North America Project Approval Letter W81XWH1590001. August 27, 2020.
DoD-Philips North America Revised Project Approval Letter (2) W81XWH1590001. September 23, 2020.
DoD-Philips North America Revised Project Approval Letter (3) W81XWH1590001. October 13, 2020.
Phlow Corporation – therapeutic.
HHS/ASPR/BARDA-Phlow Corporation Contract 75A50120000092. May 18, 2020.
Plasma Technologies – immune globulin hyperimmune manufacturing.
DOD-Plasma Technologies Contract W911QY2020004. August 14, 2020.
Protein Sciences Corporation (Sanofi) – vaccine.
HHS/ASPR/BARDA-Protein Sciences Corp Contract HHSO100201600005I. February 14, 2020.
HHS/ASPR/BARDA-Protein Sciences Corp Contract HHSO100201600005I. August 16, 2016.
Qiagen – diagnostic R&D.
HHS/ASPR/BARDA-Qiagen Contract 75A50120C00014. March 11, 2020.
Quidel – antigen assay R&D.
HHS/ASPR/BARDA-Quidel Contract 75A50120C00110. May 29, 2020.
Regeneron Pharmaceuticals – therapeutic.
HHS/ASPR/BARDA-Regeneron Other Transaction Authority Agreement HHSO100201700020C. September 21, 2017.
HHS/ASPR/BARDA-Regeneron Other Transaction Authority Agreement HHSO100201700020C-MOD0006. January 31, 2020.
HHS/ASPR/BARDA-Regeneron Other Transaction Authority Agreement HHSO100201700020C-MOD0006. (Date redacted).
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C-MOD0002. April 22, 2019.
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C-MOD0003. May 18, 2019.
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C-MOD0001. September 28, 2018.
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C-MOD0005. February 12, 2020.
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C MOD0008. June 15, 2020.
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C MOD0009. June 19, 2020.
HHS/ASPR/BARDA-Regeneron Contract HHSO100201700020C MOD0007. April 29, 2020.
Regeneron – therapeutic manufacturing.
DoD (via ATI)-Regeneron Other Transaction Authority Agreement W15QKN1691002 (contract and Technical Direction Letter). July 6, 2020.
DoD (via ATI–Regeneron Technical Direction Letter W15QKN1691002. July 6, 2020. (Version obtained November 30, 2020 from HHS FOIA Reading Room)
Regeneron – prophylactic monoclonal antibody.
DOD-Regeneron Pharmaceutical Contract W15QKN21C0014. January 12, 2021.
DOD-Regeneron Pharmaceutical Contract W15QKN21C0014-P00004. July 26, 2021.
Rigel Pharmaceuticals – therapeutic.
DOD-Rigel Pharmaceuticals Other Transaction Authority Agreement W911QY2190018. January 29, 2021.
DOD-Rigel Pharmaceuticals Other Transaction Authority Agreement W911QY2190018. January 29, 2021. (Version obtained via FOIA)
SAB Biotherapeutics – antibody diagnostic.
DOD (via ATI)-SAB Biotherapeutics Technical Direction Letter and Statement of Work – W15QKN1691002. April 18, 2020.
Sanofi – vaccine.
DOD (via ATI)-Sanofi Technical Direction Letter W15QKN1691002. July 30, 2020. (Version obtained November 30, 2020 from HHS FOIA Reading Room)
DOD (via ATI)-Sanofi Technical Direction Letter and Statement of Work RPP20-11 MCDC2011-005. December 18, 2020.
Sempulse – diagnostics technology.
DoD-Sempulse Project Approval Letter W81XWH1590001. September 23, 2020.
Sepsis Alliance – sepsis training.
HHS/ASPR/BARDA-Sepsis Alliance Contract HHSO100201900021C. January 19, 2019.
HHS/ASPR/BARDA-Sepsis Alliance Contract HHSO100201900021C-P00001. June 6, 2019.
HHS/ASPR/BARDA-Sepsis Alliance Contract HHSO100201900021C-P00002. May 7, 2020.
Sibel – diagnostics technology.
DoD-Sibel Project Approval Letter W81XWH1590001. August 27, 2020.
Siemens – immunoassay R&D.
HHS/ASPR/BARDA-Siemens Contract 75A50120C00111. May 28, 2020.
HHS/ASPR/BARDA-Siemens Contract 75A50120C00111-P00001. August 20, 2020.
HHS/ASPR/BARDA-Siemens Contract 75A50120P00102. September 17, 2020.
SIO2 – domestic pharmaceutical vials.
DOD-SIO2 Medical Contract W911NF2030003 (includes Mods 1-3). June 5, 2020.
Sonica – diagnostics.
HHS/ASPR/BARDA-Sonica Contract 75A50119C00043. August 1, 2019.
HHS/ASPR/BARDA-Sonica Contract 75A50119C00043-P00001. February 3, 2020.
SourceAmerica – face coverings.
DoD-SourceAmerica Contract W911QY20C0047 (includes Mods 1-5). April 17, 2020.
DoD-SourceAmerica Contract W911QY20C0047-P00006. November 10, 2020.
Tangen – assay.
HHS/ASPR/BARDA-Tangen Biosciences Contract 75A50120C00085. April 15, 2020.
Tasso – antibody test kits.
DoD-Tasso Serology Contract W911QY20P0158 (includes Mods 1-4). May 1, 2020. (Version obtained November 30, 2020 from HHS FOIA Reading Room)
DoD-Tasso Serology Contract W911QY20P0158-P00005 and P00006. December 3, 2020. (Version obtained via FOIA)
Texas A&M University System – expansion of manufacturing capacity (CIADM related).
HHS/ASPR/BARDA-Texas A&M University System Contract HHSO100201200002I-75A50120F33002. July 23, 2020.
HHS/ASPR/BARDA-Texas A&M University System Contract HHSO100201200002I-75A50120F33002-P0001. August 27, 2020.
HHS/ASPR/BARDA-Texas A&M University System Contract HHSO100201200002I-75A50120F33002-P0002. October 9, 2020.
Ultran Group – treatment device.
DOD (via ATI)–Ultran Group Technical Direction Letter Other Transaction Authority Agreement W15QKN1691002. September 16, 2020.
DOD (via ATI)–Ultran Group Technical Direction Letter Other Transaction Authority Agreement W15QKN1691002. September 16, 2020. (Version obtained via FOIA)
University of California at San Francisco – diagnostics technology.
University of Connecticut – vaccination technology.
HHS/ASPR/BARDA-University of Connecticut Contract 75A50120C00162. August 16, 2020.
Vaxess – spike protein vaccine manufacturing.
HHS/ASPR/BARDA-Vaxess Technologies Contract 75A50120C00160. August 6, 2020.
Vectrus-safety glasses.
DOD-Vectrus Mission Solutions Contract W911QY18D0140 (includes Mod 1). April 22, 2020.
VelocityDX – diagnostics.
DoD-VelocityDX Contract W911QY20D0031. August 12, 2020.
DoD-VelocityDX Contract W911QY20D0031. August 12, 2020. (Version obtained via FOIA)
Visby – diagnostics.
DoD-Visby Medical Contract W911QY20C0110 (includes Mod 1). September 20, 2020.
DoD-Visby Medical Contract W911QY20C0110 (includes Mod 1). September 20, 2020. (Version obtained via FOIA)
VitalConnect – vital sign threshold settings for COVID-19 patients.
HHS/ASPR/BARDA-VitalConnect Contract 75A50120C00108. May 20, 2020.
HHS/ASPR/BARDA-VitalConnect Contract 75A50120C00108-P00001. May 20, 2020.
VxBiosciences – therapeutic.
DoD-VxBiosciences Project Approval Letter W81XWH1590001. July 9, 2020.
Vyaire Medical, Inc. – ventilators.
HHS/ASPR/SNS-Vyaire Medical Contract 75A50120000049 (includes Mod 1). March 31, 2020.
Waukesha Foundry – testing swabs.
DOD-Waukesha Foundry Contract W911SR20P0009 (includes Mods 1-4). April 17, 2020.
World Enterprises – volatile organic compound detection.
DOD-World Enterprises W911SR21C0008 (includes Mods 1-3). November 13, 2020.
98Point6 – COVID-related Smartphone technology.
HHS-98Point6 Contract 75A50120C001519. October 9, 2020.
US agreements not concerning COVID-19 research, but tied to related vaccine technologies:
Moderna – Zika vaccine.
HHS/ASPR/BARDA-Moderna Contract HHS0100201600029C. September 1, 2016.
US Government licenses related to COVID-19:
NIH/NIAID-Geo Vax – vaccine primes/boosters.
NIH/NIAID-AbCellera Biologics – vaccines and therapeutics.
NIAID-AbCellera Patent License Agreement. May 4, 2020.
Europe
European Commission-AstraZeneca- vaccine.
European Commission-AstraZeneca Advance Purchase Agreement. August 27, 2020.
European Commission-Curevac – vaccine.
European Commission-Curevac Advance Purchase Agreement.
European Commission-Sanofi Pasteur and GlaxoSmithKline – vaccines.
European Commission-Sanofi Pasteur and GlaxoSmithKline Advance Purchase Agreement. September 18, 2020.
European Investment Bank-BioNTech – financing vaccines.
European Investment Bank-BioNTech Finance Contract. June 10, 2020.
European Investment Bank-Curevac – financing vaccines.
European Investment Bank-Curevac Finance Contract. June 27, 2020.
Australia
Australia-Novavax – vaccine.
Australia-Novavax Advanced Purchase Agreement. December 31, 2020.
Brazil
Fundação Oswaldo Cruz (Fiocruz)-AstraZeneca – vaccine.
Fiocruz-AstraZeneca Contract. September 8, 2020.
Canada
Canada-Novavax – vaccine.
Canada-Novavax Advanced Purchase Agreement. January 19, 2021.
China
Beijing Youfeng-Generex – vaccine.
NIVDCP-Beijing Youfeng-Generex Contract YF202011-A. November 13, 2020.
Dominican Republic
Dominican Republic-Pfizer – vaccine.
Dominican Republic-Pfizer Vaccine Term Sheet. January 19, 2021.
Israel
Israel Ministry of Health-Pfizer – vaccine.
United Kingdom
United Kingdom-Novavax – vaccine.
United Kingdom-Novavax Supply Agreement. October 22, 2020.
Inter-company Agreements Concerning COVID-19
AIM Immuno Tech-Roswell Park Cancer Institute Corporation/Roswell Park Comprehensive Cancer Center – clinical trials.
AIM Immuno Tech-Amarex – clinical trials.
AIM Immuno Tech-Amarex Project Work Order Proposal. August 4, 2020.
Amerimmune-Histogen – therapeutics.
Anixa Biosciences-OntoChem – therapeutics.
Anixa-OntoChem Collaboration Agreement. April 14, 2020.
[This link has been removed. The document is available here: https://archive.org/details/anixa-onto-chem-collaboration-agreement-14april2020 ]
AbCellera Biologics-Eli Lilly and Company – therapeutics.
AbCellera-Eli Lilly Research Collaboration and License Agreement. March 11, 2020.
GlaxoSmithKline-Vir Biotechnologies – vaccines and therapeutics.
GlaxoSmithKline-Vir Preliminary Collaboration Agreement. April 5, 2020.
Hoth Therapetics-George Washington University – smartphone-based direct virus sensing system.
Hoth-GWU Sponsored Research Agreement. September 1, 2020.
Hoth Therapeutics-Voltron Therapeutics – vaccines.
Hoth-Voltron Development and Royalty Agreement. March [redacted], 2020.
Kiromic Biopharma-Molipharma – vaccine, therapeutics, and diagnostics.
Kiromic-Molipharma Joint Venture Agreement. April 2, 2020.
Moderna-Lonza – mRNA vaccines and therapeutics.
Moderna-Lonza Global Long Term Agreement. September 4, 2020.
Nascent Biotech-Manhattan Biosolutions – vaccines and therapeutics.
Nascent-Manhattan Research Collaboration Agreement. April 30, 2020.
Novavax-Serum Institute of India – vaccine.
Novavax-Serum Institute Supply and License Agreement. July 30, 2020.
PharmaCyte Biotech-Hai Kang Life Corporation Limited – diagnostic.
PharmaCyte-Hai Kang Life License Agreement. April 2, 2020.
Pfizer – BioNTech – vaccine.
Pfizer-BioNTech Collaboration Agreement. March 17, 2020.
Articles by Children’s Health Defense (Michael Nevradakis)
https://childrenshealthdefense.org/defender/brad-miller-dod-remdesivir-defender-podcast/
https://childrenshealthdefense.org/defender/remdesivir-papers-drug-military-members-601-deaths/
https://childrenshealthdefense.org/defender/white-house-paul-friedrichs-pandemic-policy-office/
https://childrenshealthdefense.org/defender/cdc-robert-redfield-covid-origins/
https://childrenshealthdefense.org/defender/biological-weapons-biden-biodefense-strategy/
https://childrenshealthdefense.org/defender/new-covid-strain-risky-gain-of-function-research/
https://childrenshealthdefense.org/defender/whistleblower-military-covid-19-vaccine-mandate/
https://childrenshealthdefense.org/defender/dod-evidence-military-vaccine-mandate-trial/
https://childrenshealthdefense.org/defender/pfizer-johnson-pharma-funding-terrorism-iraq/
The Warren Commission Report hid the truth about the JFK assassination.
The 9-11 Commission Report hid the truth about 9-11.
Now we have this…
The outright lie that Operation Warp Speed was a “success” that “saved millions of lives” is simply no longer tenable and must be rejected.
Throughout the early rollout of COVID-19 vaccinations in the winter and spring of 2021, there was an aggressive and widespread campaign—often with the support of government public health institutions—to convince the American people to get vaccinated. However, the nuances of the vaccines’ regulatory status were unclear to most regular people. Instead, these novel mRNA vaccines were dubbed simply as “safe and effective,” with very little opportunity for patients to discuss these vaccines with their doctor and assess their individual risks and benefits. (Page 348 in the PDF)
https://oversight.house.gov/wp-content/uploads/2024/12/12.04.2024-SSCP-FINAL-REPORT.pdf
James Roguski
310-619-3055
JamesRoguski.substack.com/archive
ControlBloodSugarNaturally.com
I claim no copyright of any kind whatsoever, over any of my work, ever. Everyone is encouraged to copy any and all of it, in part, or in full, and use it for whatever purposes they wish. In fact, I would be delighted if someone were to copy this entire body of work. I encourage everyone to duplicate and mirror it in its entirety. I also encourage everyone to adapt and utilize the information in whatever manner they deem appropriate. No citation or other reference is requested or required. It would actually bring me great joy to see this information multiply exponentially and "go viral".
All content is free to all readers.
All support is deeply appreciated.








































I think they first want to kill off the old people. Old people can remember what the world used to be like. Can recognize patterns. Especially those coming of age in the sixties and seventies.
I remember the 1976 swine flu vaccination program. It rolled out fast and they put up vaccine programs in the schools and encouraged us students to go talk our parents into the vaccines. My mom said no. And that was that. Short time later people started reporting about being injured. They pulled the vax from the market. All these liabilities shields came after 1976 swine flu vaccine.
Plus, they want the young people to forget about natural immunity. Old people know about this. It’s how we stayed healthy prior to vaccines. I remember measles parties. Usually when one kid got sick, parents would let all the kids visit that kid so as to knock it out all at once and build immunity.
Folks, everything coming from our government is a lie. Food pyramid is a lie. Turn it upside down. Food pyramid will have you eating like a cow and a pig, not like a human should. Statin drugs big fat lie!
They really are trying kill us, especially the old people with memories.
God bless you James for all your tireless work this year and always! 🙏