Stop the PABS Negotiations
Pathogen Access and Benefit Sharing (PABS) is an absolutely HORRIBLE idea that must be stopped. We can still STOP the entire Pandemic Agreement by making sure that the PABS negotiations fail.
SHARE THIS LINK: https://NoPABS.com
Please watch the video below:
The next meeting of the InterGovernmental Working Group that is guiding the negotiations for the Pathogen Access and Benefit Sharing System Annex is scheduled for November 3-7, 2025.
https://apps.who.int/gb/IGWG/e/e_igwg3.html
Regardless of where you live in the world (including the United States), the negotiations for a Pathogen Access and Benefit Sharing (PABS) System will impact your life in the future.
For the latest update, please read the article below:
As of August 24, 2025, the nations involved in the PABS negotiations had submitted 78 pages of proposed text.
I have reviewed all of these documents countless times and summarized them into a 4 page Executive Summary and the 17 minute video below.
GET THE FACTS:
LISTEN TO THE AUDIOS BELOW:
Interview with Sargis Sangari on the National Security Hour on the America Out Loud Talk Network:
Twitter/X Space with Malue Montclairre:
WATCH THE VIDEO BELOW:
Backup videos:
https://www.youtube.com/watch?v=w5v-OmawOvc
https://www.bitchute.com/video/VwcroKPvgQ5z
Seriously. Please watch the video above. It provides a clear and concise summary of vast amounts of information.
READ THE DOCUMENTS:
A 4 page Executive Summary:
A 4 page Executive Summary PLUS all the official documents in one place:
NOW is the time to raise your voice in opposition to the proposed PABS System and the WHO Pandemic Agreement.
CLICK HERE TO SHARE YOUR OPINION
Your comments may be published in a future article.
If you want to work to oppose the PABS System, then contact me directly:
James Roguski 310-619-3055 (phone, text, Signal, Telegram or WhatsApp)
Executive Summary
Regardless of where you live in the world (including the United States), the negotiations for a Pathogen Access and Benefit Sharing (PABS) System will impact your life in the future.
The ongoing WHO InterGovernmental Working Group (IGWG) negotiations seek to reach an international agreement to create a global network to isolate “pathogens with pandemic potential,” identify their “genetic sequence data,” and then send them to a central BIO-HUB in Switzerland.
These pathogens and genetic information will then be shared globally with members of the WHO Collaborating Laboratory Network (WCLN) in order to create Vaccines, Therapeutics and Diagnostics (VTDs). Only those organizations and corporations that are willing to sign contracts in alignment with the details of the PABS System will be permitted to participate.
The PABS System is touted as a way of providing low-income nations with “equitable access” to “pandemic related products.” In reality, the World Health Organization seeks to control, manipulate and profit from the discovery, creation, research, development, manufacturing, regulation, distribution and administration of biological weapons-grade “pathogens with pandemic potential.”
The proposed PABS system would concentrate all the world’s most deadly pathogens into the hands of unelected, unaccountable bureaucrats.
A cartel is a partnership between two or more companies whose goal is to manipulate the market and/or the cost of goods to their advantage.
A cartel is a group of independent corporations, manufacturers, suppliers or other entities that join together to restrict competition, fix prices, rig bids, control or allocate markets, by stockpiling and marketing quotas or conduct other similar illegal activities.
Collusion occurs when entities or individuals work together to influence a market or pricing to their advantage. Acts of collusion can include price fixing and/or sharing insider information.
PABS is the new OPEC cartel:
The Organization of Pandemic Emergency Corporations
The negotiators seem to believe that the only answer is more pharmaceutical products. They want more fake diagnostics, more deadly devices, more dangerous drugs and more mRNA biological weapons masquerading as “vaccines.”
The WHO is ignoring obvious facts about their failed pharmaceutical response to COVID-19:
Using RT-PCR as a diagnostic test is a fraud.
Ventilators (along with Midazolam) killed thousands of people.
Remdesivir (Run-Death-Is-Near) also killed thousands of people.
The mRNA platform is not a vaccine, it is a biological weapon that has harmed billions of people.
They are ignoring the fact that people in nations of the “Global South” that did NOT have “equitable access” to the pharmaceutical industries’ vaccines, therapeutics and diagnostics have actually fared much better and are NOT now suffering the long-term adverse events that have reached pandemic levels among people in the “Global North.”
The proposed Pathogen Access and Benefit Sharing (PABS) System is essentially a multinational trade deal to establish a WHO controlled infrastructure for obtaining biological weapons grade pathogens, research (Gain Of Function?), development, manufacturing, and distribution of dangerous products that only benefit the Pharmaceutical Hospital Emergency Industrial Complex (PHEIC).
Participating pharmaceutical manufacturers must agree to share financial and in-kind benefits with the nations that provided the access to the pathogens and their genetic sequence data.
THIS INCENTIVIZES THE SEARCH FOR, AND THE CREATION OF, “PATHOGENS WITH PANDEMIC POTENTIAL.”
Natural remedies and pre-existing essential medications that are used by intelligent and creative health care practitioners who work outside the Pharmaceutical Hospital Emergency Industrial Complex (PHEIC) are more than adequate to prevent any and all pandemics.
The diagnostics, devices, drugs, “vaccines” and gene-altering therapies that they believe to be the way to prevent, prepare for and respond to the next pandemic, have actually caused the current pandemic of VAIDS, myocarditis, stroke, turbo cancer and sudden death.
These negotiations are not designed to prevent, prepare for, or respond to the next pandemic. The PABS negotiations are designed to incentivize the search for deadly pathogens, so that pathogens with pandemic profiteering potential can be circulated through the WHO Coordinated Laboratory Network, not for the benefit of mankind, but for the benefit of those who would profit from the diseases that they would spread through the very system that they are claiming would prevent the next pandemic.
During COVID-19, the denial of such early treatment using existing options was a crime against humanity. Now they want to reward those who find or create “pathogens with pandemic potential.”
This is NOT a path to prevent, to prepare for, or to respond to “pandemics.”
This is an attempt to create an evil cartel that will profit from the fear generated by propaganda surrounding the never-ending threat of man-made “pandemic emergencies.”
QUESTIONS:
Why is the WHO ignoring the most obvious reality, that existing natural healing solutions must be employed as soon as possible and faith in dangerous, UNTESTED, high-tech, gene-altering technologies is FRAUDULENT AND MISGUIDED.
Why in the world would we allow the WHO, which has diplomatic immunity, to be in control of all the world’s most deadly pathogens?
Who (WHO?) will be held liable for harm caused by rapidly developed, poorly tested and widely deployed “Vaccines, Therapeutics and Diagnostics?”
What defines a pathogen as being a novel “pathogen with pandemic potential?”
Why is gain-of-function research NOT being outlawed?
Will organizations and corporations be permitted to participate in the PABS System even though the country in which they are based is NOT a party to the “Pandemic Agreement?” Won’t that actually hinder research and development, rather than help it?
THE PABS NEGOTIATIONS MUST BE STOPPED.
CLICK HERE TO SHARE YOUR OPINION
“THE COBRA EFFECT”
As of August 23, 2025, a number of nations have submitted their initial text proposals for the Pathogen Access and Benefit Sharing System.
INITIAL TEXT PROPOSALS
https://apps.who.int/gb/igwg/e/e_igwg2-initial-text-proposals.html
Africa Group (3 pages)
Australia, the United Kingdom, Norway, Canada, and New Zealand (6 pages)
Bangladesh (14 pages)
Brazil (6 pages)
Central African Republic (French version) (2 pages)
China (Chinese version) (4 pages)
China (English version) (4 pages)
Colombia (4 pages)
European Union (3 pages)
Group For Equity (8 pages)
Indonesia (8 pages)
Japan (2 pages)
Malaysia (11 pages)
Russian Federation (Russian version) (10 pages)
Russian Federation (English version) (8 pages)
South Africa (10 pages)
Switzerland (5 pages)
Tunisia (French version) (13 pages)
Turkiye (1 page)
I have collected all of the text of the initial documents and I have machine translated some of the documents that were only available in French (Central African Republic and Tunisia).
The information below is provided as a go-to resource for those people around the world who really want to comprehend the details of what the WHO negotiations for the Pathogen Access and Benefit Sharing System are really all about.
CLICK HERE TO SHARE YOUR OPINION
Below are some excerpts from the documents submitted by the many nations:
PATHOGEN ACCESS
Access to PABS Materials and Sequence Information shall be subject to terms and conditions defined in a contract between the recipient and the WHO. [Africa Group]
“Pathogens with pandemic potential” [12.2] - Consider and/or address:... synthetic/AI generated sequences) [Australia, UK, Norway, Canada and New Zealand]
PABS Materials: isolated wild-type pathogens with pandemic potential, and parts thereof, modified pathogens, and any other materials derived from, generated or prepared using the PABS Materials (GAIN-OF-FUNCTION). [Brazil]
All transfers of PABS Materials within the network are subject to Standard Contract 1, which establishes the terms and conditions for sharing. [Brazil]
Sharing of Sequence Information occurs via a WHO-managed database. [Brazil]
Users of PABS Materials and revenue-generating sequences must make financial contributions. [Central African Republic]
Only individuals/entities accepting benefit-sharing commitments through legally binding contracts should have access to PABS Materials and Sequences. [Central African Republic]
In accordance with Article 12, the scope of the PABS system must be limited to "pathogens with pandemic potential." [Central African Republic]
Access to the PABS System shall be governed by Standard Material Transfer Agreements (SMTAs) and Data Access Agreements (DAAs). [Malaysia]
WHO Collaborating Centres and designated National Reference Laboratories within a WHO coordinated laboratory network for sharing PABS Material and Sequence Information shall serve as the primary hubs for the receipt, storage, and redistribution of such materials. Sequence Information shall be deposited, managed, and disseminated exclusively through a WHO database. [Malaysia]
Only authorised national laboratories that agrees to accept the Terms of Reference (ToR), shall be authorized to share PABS Materials under this Annex. [Malaysia]
PABS Sequence Information shall only be deposited in and accessed through WHO database. [Malaysia]
WHO should cover the costs of shipment incurred by developing countries. [Malaysia]
PABS Materials means isolates of PPP, parts thereof, and any materials derived from, generated or prepared using such pathogens or their parts, including attenuated strains, vectors, cell lines, reference standards, and synthetic or computationally-generated constructs arising from PABS inputs, shared by laboratories authorised by the Competent National Authority (CNA) within the WHO Laboratory Network. [South Africa]
The transfer of PABS Materials to a laboratory within the WHO Lab Network is subject to the standard WHO-Lab SMTA terms and conditions accepted upon enrollment and as updated by the COP/WHO. Execution of the WHO-Lab SMTA (and, where PSI access is sought, the DAA) is a pre-condition to access and applies with flow-down to affiliates, contractors, CROs/CMOs and any downstream recipients. [South Africa]
PABS Materials and PSI are governed under the PABS system for the specific purposes set out in the standard agreements SMTA/DAA, i.e. for pandemic prevention, preparedness and response. Access is conditional on execution of the applicable SMTA/DAA with flow-down to affiliates, contractors, CROs/CMOs and downstream recipients (“no access without obligations”). [South Africa]
All prospective recipients (persons/entities) seeking access to PABS Materials, including for research and/or for development, manufacture, authorisation or supply of vaccines, therapeutics and diagnostics (VTDs) shall execute a WHO Standard Material Transfer Agreement (SMTA) approved by the COP as a pre-condition to access (“no access without obligations”). The SMTA flows down to affiliates, contractors, CROs/CMOs, collaborators and sublicensees. [South Africa]
When technically and legally justified, the institutions that provided the biological material or associated data may be recognized as co-owners of the intellectual property or granted non-exclusive access, under a free or preferential license. [Tunisia]
BENEFIT SHARING
Each participating manufacturer shall make available to the World Health Organization, pursuant to legally binding contracts signed with the World Health Organization, rapid access targeting 20% of their real time production of safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency, provided that a minimum threshold of 10% of their real time production is made available to the World Health Organization as a donation, and the remaining percentage, with flexibility based on the nature and capacity of each participating manufacturer, is reserved at affordable prices to the World Health Organization. [Article 12]
Annual monetary contributions [Article 12]
Facilitate the manufacture and export of vaccines, therapeutics and diagnostics for pathogens [Article 12]
The granting of non-exclusive licenses to manufacturers in developing countries, for the effective production and delivery of vaccines, therapeutics and diagnostics [Article 12]
Other recipients of PABS Materials and Sequence Information must also enter into legally binding contracts with the WHO to ensure full compliance with the terms and conditions of the contracts. [Africa Group]
Access to PABS Materials and Sequence Information is contingent upon agreement to share benefits. Those benefits will be listed in the legally binding contracts as alternatives for different categories of users, and will include:
a) Annual financial contributions by commercial users based on revenues generated from pandemic-related health products developed using PABS Materials or Sequence Information;
b) Capacity-building and technical assistance;
c) Research and development cooperation;
d) Open and timely access to research data and results. Includes scientific findings, analyses, and outcomes derived from PABS use.
e) The granting of non-exclusive licenses to manufacturers in developing countries, for the effective production and delivery of vaccines, therapeutics and diagnostics; and
f) Other forms of transfer of technology as mutually agreed, including transfer of relevant knowledge, skills and technical expertise. [Brazil]
Parties shall facilitate the rapid manufacture and export of vaccines, therapeutics, and diagnostics, and ensure compliance with benefit-sharing obligations. [Brazil]
Users must report their analyses and publications to WHO, and agree to terms ensuring compliance and benefit-sharing, including contributions from revenue. [Brazil]
When revenue arises from commercialization of products which were developed thanks to the sharing or utilization of Materials or Sequence Information, participating manufacturers shall contribute a percentage of the income to benefit-sharing; [Brazil]
To ensure sufficient production during an emergency, manufacturers using PABS materials and sequences must grant manufacturing licenses to the WHO, which can then sublicense them to manufacturers in developing countries. [Central African Republic]
A fundamental element of the PABS system is that benefit sharing must ensure equitable and guaranteed access to VTDs from the initial stages of outbreaks, to prevent their development into PHEICs, and during PHEICs to prevent pandemics. This guarantee should be materialized in the form of mandatory stockpiles for developing countries. [Central African Republic]
This commitment should be applicable not only during emergency periods, but also during non-emergency periods, to build up WHO stockpiles and allow rapid access in the event of outbreaks with pandemic potential. [Central African Republic]
Ensure that benefits flow to the providing party. [China]
Benefits arising from the use of PABS Materials and Sequence Information shall be shared without undue delay… At the onset of a disease outbreak having potential to become a pandemic emergency or Public Health Emergency of International Concern (PHEIC): Through the rapid deployment of vaccines, therapeutics, and diagnostics (VTD), accompanied by immediate financial and technical support to affected developing countries to ensure equitable access and timely response as well as licensing for diversification of manufacturing to developing countries, to address shortage of supplies; [Malaysia]
Benefits arising from the use of PABS Materials and Sequence Information shall be shared without undue delay… At all times: regular building and management of national, regional or international stockpiles, and support for strengthening health systems, laboratory capacity and research and development infrastructure, and manufacturing readiness, particularly in developing countries; [Malaysia]
Benefits arising from the use of PABS Materials and Sequence Information shall be shared without undue delay… Post-pandemic period: Through sustained financial and other support to be identified aimed at building resilient health systems diversifying production and replenishing regional and global stockpiles of VTDs. [Malaysia]
Monetary contributions to the PABS Fund shall be made by recipients of PABS Materials and Sequence Information engaged in generating revenue from the direct and indirect use of PABS Materials and Sequence Information, including laboratories, institutions, researchers, developers and manufacturers of vaccines, therapeutics, and diagnostics (VTDs). They shall commit to provide such contributions by signing SMTAs and DAAs. [Malaysia]
Annual monetary contribution is based on the revenue generated from the commercialization of products and services, directly or indirectly, from using the PABS system, in accordance with rates and thresholds to be determined and set out in implementing arrangements. [Malaysia]
Funds collected pursuant to this Article shall be allocated, in a transparent and equitable manner, based on public health risk and need, with priority to countries most severely affected by the pandemic, to developing countries lacking domestic manufacturing capacity, and to WHO-coordinated mechanisms for stockpiling, logistics, and the equitable global distribution of vaccines, therapeutics, and diagnostics (VTDs) and as further determined. [Malaysia]
In distributing vaccines, medicines and diagnostics received by the PABS System, priority should be given to countries that have provided a pathogen with pandemic potential on the basis of or using which certain pandemic countermeasures have been developed. [Russian Federation]
Benefit Sharing obligations of the participating manufacturer:
(i) Annual Monetary contributions to the PABS Equity Fund calculated on global revenues from PABS-enabled products/services: [XX%] during a PHEIC/Pandemic and [YY%] interpandemic, as defined in the Revenue Calculation Annex; [South Africa]
(ii) 20% or more set aside of real-time production for WHO allocation by public-health need (with at least 10% donation and the balance at affordable prices) during pandemic emergency, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation; [South Africa]
(iii) 15% or more set aside of real time production for WHO allocation by public-health need (with at least half as donation and the balance at affordable prices) during PHIEC, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation [South Africa]
(iv) Supply to WHO stockpiles and outbreak response on request; WHO may arrange and, where appropriate, cover shipment and last-mile logistics for deliveries to developing countries;[South Africa]
(v) Pandemic emergency and PHEIC set-asides may be updated consistent with Article 12 and COP decisions; floors may be raised by COP but not lowered; [South Africa]
(vi) Licence to WHO (worldwide, non-exclusive, royalty-free) with sub-licensing to developing countries manufacturers covering background/foreground IP, regulatory data waivers/sharing, cell lines/seeds, process parameters and tacit know-how; time-bound transfers (30-day package; 60–90-day on-site support); [South Africa]
Benefit Sharing obligations of all other recipients
(i) Annual Monetary contributions: where revenues arise from PABS-enabled services/tools, apply the inter-pandemic rate above; otherwise, provide non-monetary benefits (reference materials, training, method transfer, data/tools) as specified by COP; [South Africa]
(ii) Other fit-for-purpose benefits according to recipient type (e.g., sharing validated methods, QC panels, analytics scripts), as well as cooperation to enable downstream monetary benefit-sharing where commercialisation later occurs; [South Africa]
Benefit Sharing obligations of the participating manufacturer, accessing PSI:
(i) Annual Monetary contributions to the PABS Equity Fund based on global revenues from PABS-enabled products/services: [XX%] during a PHEIC/Pandemic and [YY%] interpandemic, as defined in the Revenue Calculation Annex;[South Africa]
(ii) 20% or more set aside of real-time production for WHO allocation by public-health need (with at least 10% donation and the balance at affordable prices), during pandemic emergencies, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation; [South Africa]
(iii) 15% or more set aside of real-time production for WHO allocation by public-health need (with at least half as donation and the balance at affordable prices) during PHIEC, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation [South Africa]
(iv) Supply to WHO stockpiles and outbreak response on request; WHO may arrange and, where appropriate, cover shipment and last-mile logistics for deliveries to developing countries; [South Africa]
(v) Pandemic emergency set-asides may be updated consistent with Article 12 and COP decisions; floors may be raised by COP but not lowered; [South Africa]
(vi) Licence to WHO (worldwide, non-exclusive, royalty-free) with sub-licensing to designated developing countries manufacturers covering background/foreground IP, regulatory data waivers/sharing, cell lines/seeds, process parameters and tacit know-how; time-bound transfers (30-day package; 60–90-day on-site support); [South Africa]
Benefit Sharing obligations of all other persons/entities accessing PSI:
(i) Annual Monetary contributions: where revenues arise from PABS-enabled services/tools, apply the inter-pandemic rate above; otherwise, provide non-monetary benefits (reference materials, training, method transfer, data/tools) as specified by COP; [South Africa]
(ii) Other fit-for-purpose benefits according to recipient type (e.g., sharing validated methods, QC panels, analytics scripts), as well as cooperation to enable downstream monetary benefit-sharing where commercialisation later occurs; [South Africa]
Fair and Equitable Sharing of Benefits
IV.1. All recipients of PABS Materials and/or PSI that generate revenue (directly or indirectly) from PABS-enabled products or services shall contribute annual monetary benefit-sharing to the PABS Equity Fund, calculated on global revenues in accordance with a COP-adopted Revenue Calculation Annex. Rates shall be [XX%] during a PHEIC/Pandemic and [YY%] inter-pandemic, as applicable. Recipients shall report revenues to WHO (with audit-light verification). [South Africa]
IV.2. All recipients shall provide non-monetary benefits appropriate to their role, in addition to monetary contributions. Manufacturers accessing PABS Materials and/or PSI shall, as a condition of the SMTA/DAA, provide at minimum: [South Africa]
(a) set-asides of real-time pandemic emergencies and PHEICs; [South Africa]
(b) support to WHO stockpiles and outbreak response; [South Africa]
(c) licences to WHO with sub-licensing to designated developing countries manufacturers, including transfer of cell lines/seeds, regulatory data, process parameters and tacit knowhow with time-bound milestones; and [South Africa]
(d) training, method transfer and access to reference standards/QC panels. [South Africa]
Non-manufacturing recipients (e.g., academic labs, repositories, service providers) shall provide fit-for-purpose non-monetary benefits (e.g., validated methods, analytics scripts, data tools, training) and cooperate to enable downstream monetary benefit-sharing where later commercialisation occurs. All obligations flow down to affiliates, contractors, CROs/CMOs and sublicensees and are recorded in COP-mandated tracking systems. [South Africa]
Non-manufacturing recipients (e.g., academic labs, repositories, service providers) shall provide fit-for-purpose non-monetary benefits (e.g., validated methods, analytics scripts, data tools, training) and cooperate to enable downstream monetary benefit-sharing where later commercialisation occurs. All obligations flow down to affiliates, contractors, CROs/CMOs and sublicensees and are recorded in COP-mandated tracking systems. [South Africa]
The distribution and use of monetary and non-monetary benefits shall occur under COP direction, administered by the WHO Secretariat. The COP establishes allocation principles prioritising public-health needs with particular attention to developing countries, regional balance and surge capacity. [South Africa]
Tunisia maintains that any country that has contributed to the PABS system by sharing biological resources or genetic data has the right to:
receive a guaranteed minimum quota of products developed from these resources (in kind or otherwise);
be included in manufacturing partnerships, based on its national or regional industrial capabilities;
request technical or financial support to upgrade its pharmaceutical production capacities. [Tunisia]
A multilateral funding mechanism should be established within the PABS system, funded in particular by mandatory or voluntary contributions from beneficiary private entities. [Tunisia]
Any manufacturer that has accessed pathogens must commit to returning a portion of the products developed or profits to the country of origin. [Tunisia]
Any commercial exploitation of a product derived from PABS resources must be subject to prior notification to the supplier country and include a negotiated sharing of profits or royalties, in accordance with the SMTA. [Tunisia]
Expected benefits include: Funding for P3-level national laboratories [Tunisia]
Expected benefits include: Preferential access to health products (vaccines, tests, treatments) (details: percentage, free of charge) [Tunisia]
This sharing includes benefits: Monetary (pecuniary): mandatory annual contributions to the PABS Fund, proportional royalties in the event of commercialization; Non-monetary (non-pecuniary): priority access to health products, technology transfer, scientific co-development, and national capacity building. [Tunisia]
Any entity benefiting from the PABS system, whether public or private, is required to include in the Standard Material Transfer Agreements (SMTA) a clause stipulating:
the provision of a specified share of final products (vaccines, treatments, diagnostics) to supplier countries, free of charge or at an affordable cost, particularly in the event of a public health emergency;
the prioritization of low- and middle-income countries, particularly those in the Global South, in distribution strategies during pandemic situations. [Tunisia]
Countries that have provided a pathogen or its genetic data that caused the pandemic emergency benefit from a priority quota of health products, equivalent to at least 10% of the available global production of each type of derived product, delivered within 30 days of their operational availability. [Tunisia]
GOVERNANCE (BUREAUCRACY)
The Conference of the Parties (COP) to the WHO Pandemic Agreement shall establish a dedicated PABS Secretariat, which shall be responsible for managing and maintaining global registries of PABS Materials and Sequence Information; [Article12]
Administration and coordination of the PABS System shall be undertaken by the World Health Organization under the guidance of the Conference of the Parties, including review by a subsidiary body established by it. [African Group]
WHO Coordinated Laboratory Network (WCLN) is an international network of authorized laboratories managed by WHO, governed by the PABS System under the supervision of the Conference of the Parties (COP). Membership requires meeting designated criteria and compliance with contractual terms. [Brazil]
The WHO Coordinated Laboratory Network (WCLN) will be established under WHO coordination, linking authorized national laboratories that meet the criteria and agree to abide by the Terms of Reference (ToR), which are legally binding. [Brazil]
The PABS System shall be coordinated and administered by WHO, under the authority of the Conference of Parties (COP) [Brazil]
WHO shall coordinate and administer the PABS system, under the supervision of the Conference of the Parties. [Central African Republic]
The PABS System shall operate under a multi-tiered governance structure. [Malaysia]
WHO shall determine allocation and distribution of VTDs, under guidance of COP, including through the Global Supply Chain and Logistics (GSCL) Network, to ensure that donated and purchased VTDs are distributed equitably based on public health need. [Malaysia]
WHO under the guidance and authority of Conference of Parties to the WHO Pandemic Agreement shall provide the infrastructure for the PABS System, ensuring it is functional, transparent, and accessible to all Parties. [Malaysia]
WHO shall be responsible for managing and maintaining global registries of PABS Materials and Sequence Information; coordinating the collection, allocation, and disbursement of monetary and non-monetary benefit-sharing contributions; and overseeing and verifying compliance with Standard Material Transfer Agreements (SMTAs), Data Access Agreements (DAAs), and benefit-sharing commitments. [Malaysia]
WHO shall record and monitor all accesses, transfers and uses of PABS Materials and Sequence Information, tracking each item from its source through to final use and disposition. [Malaysia]
WHO shall coordinate and oversee the establishment of transparent timelines and mechanisms to track the provision, allocation, and delivery of such benefits, including VTDs, in accordance with this Annex. [Malaysia]
Such contributions shall be monitored, enforced, and reported by WHO to ensure compliance with the provisions of this Annex and to facilitate equitable benefits haring across all participating States. [Malaysia]
Each State Party shall designate National Focal Point (NFP) or other competent national authorities, as appropriate, to authorize the release and facilitate the transfer of PABS Materials and Sequence Information in accordance with the provisions of this Annex. [Malaysia]
The Conference of the Parties (COP) to the WHO Pandemic Agreement shall establish a dedicated PABS Secretariat, which shall be responsible for managing and maintaining global registries of PABS Materials and Sequence Information; coordinating the collection, allocation, and disbursement of monetary and nonmonetary benefit-sharing contributions; and overseeing and verifying compliance with Standard Material Transfer Agreements (SMTAs), Data Access Agreements (DAAs), and benefit-sharing commitments. The PABS Secretariat shall exercise its functions in full respect of national laws, sovereignty, and applicable international obligations, working in partnership with the competent authorities of Provider States. [Malaysia]
The Conference of the Parties (COP) to the WHO Pandemic Agreement shall inter alia approve strategic priorities, operational policies, and budgetary allocations for the PABS System; ensure the timely and equitable distribution of vaccines, therapeutics, and diagnostics (VTDs) based on public health risk and equity considerations; and review and take decisions on the annual PABS implementation and performance report prepared by the PABS Secretariat. [Malaysia]
Day-to-day operations of the PABS System shall be coordinated and administered by the WHO, including conducted through WHO-designated laboratories, comprising national and regional reference laboratories responsible for the receipt, handling, and redistribution of PABS Materials; WHO global databases for the secure storage and dissemination of PABS Sequence Information; and distribution centres responsible for the storage and delivery of stockpiled or donated vaccines, therapeutics, and diagnostics (VTDs). All such operational laboratories shall, as a condition of designation and continued participation, comply with internationally recognized biosafety and biosecurity standards, transparency requirements, and reporting obligations as set forth in this Annex. [Malaysia]
Under the authority of COP, WHO acting as PABS Secretariat, shall ensure the PABS System is functional, transparent, accountable to all Parties. WHO’s role will typically include:
a. Establish and maintain the central governance and coordination platform for the PABS System, including oversight of registries, data standards, and compliance mechanisms.
b. Act as the neutral intermediary between Provider (who share PABS Materials and Sequence Information) and Recipients (e.g., manufacturers, research institutions) to ensure equitable access and benefit-sharing.
c. Develop, host, and maintain the WHO PABS Sequence Information Database – a secure, standardized global database for PABS Sequence Information, accessible to Parties and registered users that have accepted DAAs.
d. Manage digital registries and traceability systems, including Digital Object Identifiers (DOIs) for samples and sequence information, to track material and sequence use and enforce benefit-sharing obligations.
e. Provide secure digital platforms for Standard Material Transfer Agreements (SMTAs) and Data Access Agreements (DAAs).
f. Coordinate WHO-led global stockpiling and distribution mechanisms for vaccines, diagnostics, and therapeutics (VTDs) during pandemic emergency or PHEICs and to prevent such occurrences.
g. Support rapid and secure shipment logistics for PABS Materials, especially for emergency response and R&D needs.
h. Provide training, infrastructure funding, and technical support to national laboratories, sequencing centres, and manufacturing hubs (particularly in developing countries).
i. Facilitate technology transfer and local production capacity-building to promote equitable access and self-reliance. [Malaysia]
WHO shall establish and maintain a publicly accessible roster of all manufacturers participating in the PABS System. This roster shall include, at a minimum:
a. Information on each manufacturer’s production capacity for vaccines, therapeutics, and diagnostics (VTDs);
b. Details of their monetary and in-kind contributions, including dose donations and licensing arrangements; and
c. Records of their compliance with the obligations set forth in this Annex, including adherence to benefit-sharing, licensing, and reporting requirements. [Malaysia]
The Conference of Parties (COP) to the World Health Organisation (WHO) Pandemic Agreement is the ultimate decision-making authority with respect to the Pathogen Access and Benefit Sharing (PABS) system. WHO coordinates and administers the PABS System under the authority and guidance of the COP. [South Africa]
The COP adopts, amends and periodically reviews the instruments and agreements used to implement the PABS System, including SMTAs/DAAs, Technical Standards (e.g., lab safety, transport of biological materials, cyber-biosecurity and persistent identifiers), a Revenue Calculation Annex and a Procurement Policy. The COP may complement, amend or replace instruments to improve implementation. [South Africa]
For legal certainty, the PABS Instrument forms an integral part of the Agreement and shall not weaken essential elements, namely: scope including DSI/derivatives; conditional access via standard agreements with flow-down obligations; minimum non-monetary and monetary benefit-sharing floors; transparency; and compliance/enforcement. The COP establishes and oversees a PABS Equity Fund. [South Africa]
Just as in the case of the PIP framework model, the WHO Secretariat acts as the PABS Secretariat. The staffing and other resources are to scaled up stage by stage, taking into account the development and unfolding of functionalities within the PABS system, as well as the scale of transactions and the revenue available for such purposes. [South Africa]
Core operational functions include:
(a) coordinating the WHO Laboratory Network and operating the WHO Repository for PABS Sequence Information (WHO PSI Repository) with a first-deposition rule and controlled mirroring;
(b) maintaining public dashboards on setasides, deliveries, prices and monetary contributions, and a public roster of participating manufacturers;
(c) administering standard SMTAs/DAAs and ensuring flow-down to affiliates and contractors;
(d) implementing the Procurement Policy;
(e) arranging shipment and last-mile logistics support for deliveries to developing countries as approved by the COP;
(f) conducting independent/regular monitoring and evaluation and supporting an operational simulation exercise for continuous improvement; [South Africa]
it is proposed that a network of national laboratories authorised by their governments or by their Competent National Authority (CNA) be set up for the sharing of pathogens with pandemic potential (PPP), coordinated by WHO. To be a part of the WHO coordinated laboratory network (WHO lab network), national authorised laboratories shall meet Conference of the Parties (COP)-adopted criteria, accept specific Terms of Reference detailing tasks, and execute the standard WHO-Lab Standard Materials Transfer Agreement SMTA (and the DAA where PABS Sequence Information (PSI) access is sought) as a pre-condition to access, with flow-down to affiliates, contractors, CROs/CMOs and any downstream recipients (one-time execution unless a Party requires a signed agreement). PABS Sequence Information (PSI) generated shall be deposited first in the WHO PSI Repository pursuant to the DAA; onward sharing within the Network is recorded in a COP-mandated tracking system using persistent identifiers. [South Africa]
To achieve these objectives, it is essential to establish a WHO PABS Sequence Information (PSI) Repository and authorise other repositories or databases to host and disseminate PSI, under contracts with WHO and accountable to the COP. [South Africa]
The WHO PSI Repository shall ensure that PABS Sequence Information (PSI) generated from PABS Materials is shared through a single WHO-designated point of first deposition and made accessible in accordance with COP-adopted instruments, rather than at the discretion of individual repositories or databases. Accessibility is provided through registered user accounts subject to a COP-approved Data Access Agreement (DAA). The WHO PSI Repository and authorised repositories shall implement certain requirements in particular user registration, acceptance of the DAA (See III.3 below) as well as ensure the PSI is sufficiently labelled as PABS sequence information, using persistent unique identifiers (e.g., DOIs), which shall be tracked via audit logs. [South Africa]
Advisory group comprising experts from Parties (fair representation from developed and developing countries) shall be established to advise WHO regarding the operations of the PABS System and make recommendations for decision-making by the COP. [South Africa]
Compliance of the WHO Lab Network with the SMTA shall be monitored by the Director General. In the event of non-compliance with the SMTA and the relevant ToR, appropriate action will be taken, including suspension or revocation of the WHO designation from the laboratory. [South Africa]
The PABS system is coordinated by WHO, in collaboration with relevant international organizations and stakeholders, in accordance with their respective mandates. [Tunisia]
Partner international organizations are selected based on objective criteria defined by a PABS Governance Committee. [Tunisia]
The WHO and its partners coordinate logistics, including transportation, distribution, and technical support for recipient countries. [Tunisia]
In the event of a declaration by the WHO of a Public Health Emergency of International Concern (PHEIC), the following PABS obligations are automatically activated and without preconditions: Temporary exemption from any export restriction or health protectionism that undermines equity of access. [Tunisia]
The Parties recognize the World Health Organization (WHO) as the central coordinating authority of the PABS system, responsible for its operational management, monitoring, strategic oversight, and support to the Parties. [Tunisia]
The World Health Organization shall establish a permanent PABS coordination unit, responsible for:
the centralized management of Standard Material Transfer Agreements (SMTAs);
monitoring the circulation of pathogens and their associated data;
overseeing the PABS digital platform;
coordinating with the Parties and relevant stakeholders. [Tunisia]
A secure digital platform is established and administered by the WHO to:
facilitate the registration, signing, and monitoring of SMTAs;
ensure the traceability of PABS resources and derived products;
ensure transparency of user commitments and accountability;
enable public access to non-sensitive scientific data, in compliance with national data protection and biosafety legislation. [Tunisia]
Tunisia recommends that the WHO establish a dedicated governance body for the PABS system, composed in a balanced and multi-stakeholder manner. This body includes representatives from each WHO region, with equal representation, with a rotating chair alternating between high-income countries and low- and middle-income countries. [Tunisia]
The Parties undertake to undertake a review of their national or regional legal frameworks relating to access to biological resources and benefit-sharing (ABS), to ensure their compatibility with this PABS system. [Tunisia]
Article 12 of the “Pandemic Agreement”
https://apps.who.int/gb/ebwha/pdf_files/WHA78/A78_R1-en.pdf
Article 12 Pathogen Access and Benefit-Sharing System
Section A – Scope (Article 12.1)
1. Recognizing the sovereign right of States over their biological resources and the importance of collective action to mitigate public health risks, and underscoring the importance of promoting the rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes, the Parties hereby establish a multilateral system for safe, transparent, and accountable access and benefit-sharing for PABS Materials and Sequence Information, the “WHO Pathogen Access and Benefit-Sharing System” (hereinafter the “PABS System”), to be developed pursuant to paragraph 2 of this Article.
Section B – Elements to be developed in the PABS System (Article 12.2 and 12.5)
2. The provisions governing the PABS System, including definitions of pathogens with pandemic potential and PABS Materials and Sequence Information, modalities, legal nature, terms and conditions, and operational dimensions, shall be developed and agreed in an instrument in accordance with Chapter III (hereinafter the “PABS Instrument”) as an annex. The PABS Instrument shall also define the terms for the administration and coordination of the PABS System by the World Health Organization. For the purposes of the coordination and operation of the PABS System, the World Health Organization shall collaborate with relevant international organizations and relevant stakeholders. All elements of the PABS System shall come into operation simultaneously in accordance with the terms of the PABS Instrument.
Section C – Traceability and open access to data (Article 12.3)
3. Taking into account the differences in the use of PABS Materials and Sequence Information, the development of a safe, accountable and transparent PABS System shall address traceability measures and open access to data.
Section D – Consistency with Nagoya Protocol (Article 12.4)
4. Having regard to Article 4.4 of the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits arising from their utilization to the Convention on Biological Diversity (hereinafter the “Nagoya Protocol”), the PABS Instrument shall be consistent with, and not run counter to, the objectives of the Convention on Biological Diversity and the Nagoya Protocol, recognising that nothing in this paragraph creates obligations under these instruments for non-Parties thereto.
5. The PABS Instrument referred to in paragraph 2 of this Article, shall contain provisions regarding, inter alia, the following:
(a) the rapid and timely sharing of PABS Materials and Sequence Information and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits, both monetary and non-monetary, including annual monetary contributions, vaccines, therapeutics and diagnostics arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes;
(b) modalities, terms and conditions on access and benefit sharing that provide legal certainty;
(c) implementation in a manner to strengthen, facilitate and accelerate research and innovation, as well as the fair and equitable sharing and distribution of benefits;
(d) development and implementation in a manner:
(i) complementary to, and not duplicative of, the access and benefit sharing measures and obligations of the Pandemic Influenza Preparedness Framework and other relevant international access and benefit sharing instruments, where applicable; and
(ii) to ensure that each Party reviews and, as it deems appropriate, aligns its national and/or regional access and benefit sharing measures applicable to PABS Materials and Sequence Information within the scope of the PABS Instrument, so that measures that are contrary to, or inconsistent with, or duplicative of, the PABS Instrument will not be applied upon entry into operation of all elements of the PABS System.
(e) implementation consistent with applicable international law and with applicable national and/or domestic law, regulations and standards related to risk assessment, biosafety, biosecurity and export control of pathogens, and data protection; and
(f) implementation in a manner to facilitate the manufacture and export of vaccines, therapeutics and diagnostics for pathogens covered by the PABS Instrument.
Section E – Provisions in the event of pandemic emergency (Article 12.6)
6. The PABS System, as set out in the Annex referred to in paragraph 2 of this Article, shall provide, inter alia, that in the event of a pandemic emergency, as determined in accordance with Article 12 of the International Health Regulations (2005):
(a) each participating manufacturer shall make available to the World Health Organization, pursuant to legally binding contracts signed with the World Health Organization, rapid access targeting 20% of their real time production of safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency, provided that a minimum threshold of 10% of their real time production is made available to the World Health Organization as a donation, and the remaining percentage, with flexibility based on the nature and capacity of each participating manufacturer, is reserved at affordable prices to the World Health Organization; and
(b) the distribution of these vaccines, therapeutics, and diagnostics shall be on the basis of public health risk and need, with particular attention to the needs of developing countries, and the Global Supply Chain and Logistics Network referred to in Article 13 may be used to this end.
Section F – Provisions in the event of a public health emergency of international concern (Article 12.7)
7. The PABS Instrument shall also include benefit sharing provisions, in the event of a public health emergency of international concern as determined in accordance with Article 12 of the International Health Regulations (2005), including options regarding access to safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the public health emergency of international concern, pursuant to legally binding contracts signed by participating manufacturers with the World Health Organization.
Section G – Additional benefit-sharing provisions (Article 12.8)
8. The PABS Instrument shall also include additional benefit sharing provisions to be set out in legally binding contracts signed with the World Health Organization, including options for:
(a) Capacity-building and technical assistance;
(b) research and development cooperation;
(c) facilitating rapid access to available vaccines, therapeutics and diagnostics with a view to responding to public health risks and events in the context of Article 13.3 of the International Health Regulations (2005);
(d) the granting of non-exclusive licences to manufacturers in developing countries, for the effective production and delivery of vaccines, therapeutics and diagnostics; and
(e) other forms of transfer of technology as mutually agreed, including transfer of relevant knowledge, skills and technical expertise.
Section H – Other elements for effective operationalization of the PABS System (Article 12.9)
9. This Article is without prejudice to consideration of other elements for the effective operationalization of the PABS System in a fair, transparent, accountable and equitable manner.
AFRICA GROUP
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Africa_Group.pdf
DRAFT – CONFIDENTIAL
Initial Submission of the Africa Group on the Pathogen Access and Benefit-Sharing (PABS) Instrument August 2025
In response to the invitation by the Bureau of the IGWG to submit initial text proposals, in particular the elements to be addressed by the Pathogen Access and Benefit Sharing (PABS) Annex, the Africa Group submits preliminary input to support the work of the Bureau to develop a draft outline of elements to be addressed by the PABS Annex. This preliminary input is without prejudice to the position of the Africa group in future negotiations. The Africa Group reserves its rights to change, nuance, amend these proposals as it sees fit, and to submit new proposals.
The Africa Group reaffirms its strong commitment to the timely and effective conclusion of the PABS Annex to enable entry into force of the Pandemic Agreement. Recognizing the need to safeguard public health while upholding the sovereign rights of States over their genetic resources, as reflected in the Common African Position on Pandemic Prevention, Preparedness and Response (PPPR). The Africa Group supports a multilateral system that ensures equitable, transparent, and predictable benefit-sharing and underscores the importance of legal coherence with existing international frameworks, including PIP Framework, the Convention on Biological Diversity, and the Nagoya Protocol.
The elements set out below derive from Article 12 of the Pandemic Agreement and are essential to develop a well-functioning PABS System.
General
1) Object and Purpose: The provision on object and purpose seeks to establish the functional scope and legal intent of the Annex. Since the PABS System is central to ensuring equity under the WHO Pandemic Agreement (Article 12), the object and purpose must clearly reflect its dual mandate, namely: i) rapid, safe and timely access to PABS Materials and Sequence Information, and on equal footing, ii) the rapid, timely, fair and equitable sharing of benefits, especially with developing countries that are the main source of these materials.
2) Use of Terms: The following key terms must be defined to prevent ambiguity and provide operational clarity, legal certainty and enforceability: i) “PABS Materials and Sequence Information”; ii) “Pathogens with Pandemic Potential”; iii) “Provider” and “Recipient” of PABS Materials and Sequence Information; iv) “Participating Manufacturer”, “User”, “Commercial DSI User”; v) “Standard Material Transfer Agreement”.
3) Scope: This Annex applies i) to all PABS Materials and Sequence Information shared through the PABS System for public health purposes related to pandemic prevention, preparedness, and response, as well as to all benefit-sharing obligations and entitlements arising from the access, use or transfer of PABS Materials and Sequence Information under the PABS System; ii) to all state parties to the Pandemic Agreement, Providers and Recipients of such PABS Materials and Sequence Information, Participating Manufacturers, the WHO Secretariat, and relevant regional health governmental institutions; and iii) during inter-pandemic periods, PHEICs, and pandemic emergencies as defined under the IHR.
Fundamental Principles
The proposed foundational principles which form the normative basis of the entire PABS System are the following:
1) Sovereign rights of States over their biological resources: Principles of sharing would be based on the sovereign rights of Member States over their biological resources and in a coordinated manner through designation of national focal point.
2) Rapid and timely access to PABS Materials and Sequence Information, and on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising therefrom (nonmonetary and monetary benefits, including annual contributions).
3) Primacy of mutual trust and cooperation, accountability, and transparency in the PABS System.
4) Ethical use, integrity, and traceability of PABS Materials and Sequence Information.
Access and Use
1) Access to PABS Materials and Sequence Information shall be subject to terms and conditions defined in a contract between the recipient and the WHO.
2) Contracts between the WHO and a Participating Manufacturer shall be public and shall contain provisions relating to the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of such materials/information.
3) Other Recipients of PABS Materials and Sequence Information must also enter into legally binding contracts with the WHO to ensure full compliance with the terms and conditions of the contracts.
Traceability measures of PABS Material
The WHO shall establish a tracing and tracking mechanism and rules of procedures governing access to PABS materials and Sequence Information, guaranteeing transparency and traceability.
Metadata and identifiers associated with PABS Sequence Information, including unique labels that trace sequences to their country of origin and the PABS System, shall not be removed or altered, to ensure traceability, scientific utility, and transparency in the use of pathogen resources.
Benefit Sharing
Pandemic emergency
In the event of a pandemic emergency, each participating manufacturer shall make available to the World Health Organization, pursuant to legally binding contracts, rapid access targeting 20% of their real time production of safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency, provided that a minimum threshold of 10% of their real time production is made available to the World Health Organization as a donation, and the remaining percentage, with flexibility based on the nature and capacity of each participating manufacturer, is reserved at affordable prices to the World Health Organization. Distribution of benefits shall be on the basis of public health risk and need, with particular attention to the needs of developing countries, and within strict time limits.
Public health emergency of international concern
Contracts between the WHO and Participating Manufacturers shall include benefit sharing provisions guaranteeing that, in the event of a public health emergency of international concern (PHEIC) as determined in accordance with International Health Regulations (2005), they shall provide rapid access to safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the public health emergency of international concern.
Additional benefits
Contracts between the WHO and Participating Manufacturers shall also include provisions regarding the sharing of the following additional benefits by the Participating Manufacturer, including: i) annual monetary contributions to the PABS system to support initiatives for transfer of technology and know-how, research and development, scientific and research collaborations, and laboratory capacity strengthening; ii) provision of vaccines, therapeutics and diagnostics arising from the sharing and/or use of PABS Materials and Sequence Information for public health purposes, 10% free of charge and 10% at not-for-profit rate during the public health emergency of international concern; iii) technology transfers, co-development, and R&D.
Governance, Compliance, Accountability and Enforcement
Administration and coordination of the PABS System shall be undertaken by the World Health Organization under the guidance of the Conference of the Parties, including review by a subsidiary body established by it.
Others
1) Settlement of Disputes: There shall be a dispute settlement mechanism under this Annex pursuant to the provisions of the WHO Pandemic Agreement.
2) Entry into force: The Annex shall enter into force simultaneously with the WHO Pandemic Agreement as an Integral part thereof.
3) Cross referencing provisions of the Nagoya Protocol relevant to traditional knowledge associated with PABS Materials and Sequence Information. ***
AUSTRALIA, THE UNITED KINGDOM, NORWAY, CANADA AND NEW ZEALAND
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/AUS-UK-NOR-CAN-NZ.pdf
PABS Annex: Submission to inform the outline of elements for IGWG
This submission, provided on behalf of Australia, the United Kingdom, Norway, Canada, and New Zealand is intended to inform the work of the open-ended Intergovernmental Working Group on the WHO Pandemic Agreement (IGWG) to draft and negotiate the Pathogen Access and Benefit-Sharing (PABS) Annex. The submission aims to support the IGWG Bureau to develop a draft outline of elements to be addressed by the PABS Annex. It sets out five key elements expected to be addressed linked to Article 12 of the WHO Pandemic Agreement, issues the IGWG will need to consider and/or address, and expertise needed to inform these considerations (refer Attachment 1).
Please note that the input below is without prejudice to further input and proposals, including for legal text, that the above mentioned countries may decide to submit subsequently during the IGWG process to develop the PABS Annex.
1. Scope, principles and definitions
Scope: The “WHO Pathogen Access and Benefit-Sharing System” (“PABS System”) is a “multilateral system” providing for the “rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (“PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes.” [12.1] -
Consider and/or address: links as appropriate to existing Access and Benefit-Sharing mechanisms, see “4. Consistency, complementarity and non-duplication”.
Principles underpinning the PABS System:
Recognition of “the sovereign right of States over their biological resources and of the importance of collective action to mitigate public health risks” [12.1]
“[S]afe, transparent, and accountable access and benefit-sharing” [12.1] “that provide[s] legal certainty” [12.5(b)] and “strengthen[s], facilitate[s] and accelerate[s] research and innovation, as well as the fair and equitable sharing and distribution of benefits” [12.5(c)], and “facilitate[s] the manufacture and export of vaccines, therapeutics and diagnostics” (VTDs) [12.5(f)]
Development and implementation to complement and not duplicate access and benefit-sharing (ABS) measures and obligations, including the Pandemic Influenza Preparedness (PIP) Framework and other international instruments [12.5(d)]
Defined terms:
“Pathogens with pandemic potential” [12.2] - Consider and/or address: factors (e.g., transmissibility, geographic spread, virulence, availability of effective countermeasures, origins, and mutation potential) and process to pre-define and/or identify specific pathogens/pathogen families, including to future-proof the term (e.g., capturing pathogen X and synthetic/AI generated sequences) • “PABS materials” and “PABS sequence information” [12.1]
- Consider and/or address: separate definitions of materials (samples vs. isolates) and sequence information; alignment with other relevant instruments, whilst avoiding new definitions that could impact on those instruments; future proofing to avoid limiting scientific discovery and technological advancements; implications of excluding or including certain materials and information (e.g., associated clinical and epidemiological metadata) in both human, animal and environmentally derived sequences to capture zoonotic pathogens and consider how these different samples/sequence data are managed
“Participating manufacturer” [12.6(a) and footnote 15]
- Consider and/or address: link to legally binding contracts per 12.6(a) and 12.7 and any factors the definition should encompass
Other terms that may need to be defined: e.g., related to institutions, organizations and entities engaged in the PABS system, and potential terms used in “Access” and “Benefit-sharing” provisions, such as “rapid and timely” [12.1 and 12.5(a)]
2. Access
Provisions for “rapid and timely sharing of PABS Materials and Sequence Information” [12.1, 12.5(a)] including to “strengthen, facilitate and accelerate research and innovation” [12.5(c)]
Consider and/or address:
Current practices, functionality, capacities, and costs associated with sharing/accessing different pathogens with pandemic potential (i.e., databases for sequence data and associated meta-data; and laboratories for materials, including relevant handling and transfer practices to ensure biosafety and biosecurity)
Provisions for Parties for “rapid and timely” sharing obtained through routine surveillance and during acute events under the International Health Regulations
Accountability, transparency, traceability measures and open access to data [12.3] and legal certainty over data ownership, rights and obligations for institutions, organizations, entities or individuals sharing and accessing data. This should take into account, inter alia:
differences in sharing and use of PABS Materials and Sequence Information ▪ requirements from regulatory agencies that regulate sharing (including cross border) and access for laboratories, product developers and manufacturers including for product authorization,
lessons learned from other ABS systems’ access and traceability arrangements such as under the International Treaty on Plant Genetic Resources for Food and Agriculture and the Cali Fund,
potential use of existing databases (e.g., IP databases, clinical trial registries) to address traceability for use of PABS materials or sequence information for product development, or alternative strategies to address accountability, and
incentives for research and innovation
Other modalities to ensure access provisions satisfy the objectives of the PABS System, can be effectively operationalised, and are future-proofed (e.g., as genomic sequence data related analysis shifts to bioinformatic and artificial intelligence approaches)
3. Benefit-sharing
Provisions for “rapid, timely, fair and equitable sharing of … monetary and non-monetary [benefits] … arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes” [12.1, 12.5(a)]
Pursuant to legally binding contracts between WHO and participating manufacturers including: - in pandemic emergencies, rapid access to a 20% target of real time production of safe, quality and effective VTDs for the pathogen causing the pandemic emergency (minimum 10% available to WHO as donation; remaining percentage reserved at affordable prices, with flexibility based on nature and capacity of each participating manufacturer) to be distributed on the basis of public health risk and need, potentially through the Global Supply Chain and Logistics Network [12.6(a) and (b)] - during a PHEIC, options regarding access to safe, quality and effective VTDs for the pathogen causing the PHEIC [12.7]
- additional benefit-sharing provisions, “including options for: capacity building and technical assistance; research and development cooperation; facilitating rapid access to available [VTDs] with a view to responding to public health risks and events … ; the granting of non-exclusive licences to manufacturers in developing countries, for effective production and delivery of [VTDs]; and other forms of transfer of technology as mutually agreed [refer footnote 8 in the WHO Pandemic Agreement], including transfer of relevant knowledge, skills and technical expertise” [12.8]
- Consider and/or address:
Provisions regarding the framework for legally binding contracts between WHO and participating manufacturers for benefit-sharing and options for benefit-sharing, including whether model clauses form an appendix or are adopted by the Conference of the Parties
Provisions regarding the negotiation of contracts with participating manufacturers that provide legal certainty, consider the manufacturer’s nature and capacity, and increase the likelihood of manufacturers electing options for product access and other benefit-sharing provisions without disincentivizing product development or conclusion of legally binding contracts between WHO and participating manufacturers, and
Provisions regarding a framework for the distribution of VTDs, including the process and criteria to determine ‘public health risk and need’, and the role of WHO, other relevant international organizations, and use of the Global Supply Chain and Logistics Network
“[A]nnual monetary contributions” [12.5(a)] - Consider and/or address:
Provisions regarding the sharing of monetary benefits, including annual monetary contributions, and consider what form non-monetary benefits might take
Criteria for quantification and terms of contributions
Governance of contributions once received by the PABS system including transparency, reporting, and scope for spending/allocation – linking to “5. Governance and general provisions
Overall - Consider and/or address:
Other modalities to ensure benefit-sharing provisions satisfy the objectives of the PABS System, can be effectively operationalised, and are future-proofed (e.g., incentivizing entities’ engagement including those entities that would share and/or use PABS Materials and Sequence Information such as for clinical diagnostic purposes and other types of routine medical testing through to R&D)
4. Consistency, complementarity and non-duplication
International ABS instruments: “[T]he PABS [Annex] shall be consistent with, and not run counter to, the objectives of the Convention on Biological Diversity (CBD) and the Nagoya Protocol” [12.4]. It is developed and implemented in a manner “complementary to, and not duplicative of, the [ABS] measures and obligations of the [PIP] Framework and other relevant international [ABS] instruments where applicable” [12.5(d)(i)]
- Consider and/or address:
Consistency with CBD and Nagoya Protocol objectives, so the PABS Annex can be considered a specialized international instrument for the purposes of Article 4.4 of the Nagoya Protocol
Complementarity and no duplication with the PIP Framework including to ensure: legal certainty for all who share and access PIP biological materials and PABS Materials and Sequence Information; and complementarity between manufacturers’ legally binding contracts for benefit-sharing under the PABS System and SMTA2s under the PIP Framework
Complementarity, no duplication and legal certainty in relation to other applicable international ABS instruments, including the CBD’s Multilateral Mechanism for Digital Sequence Information
National and/or regional ABS measures:
“[E]ach Party reviews and, as it deems appropriate, aligns its national and/or regional [ABS] measures applicable to PABS Materials and Sequence Information within the scope of the PABS [Annex], so that measures that are contrary to, or inconsistent with, or duplicative of, the PABS [Annex] will not be applied upon entry into operation of all elements of the PABS System” [12.5(d)(ii)]
- Consider and/or address:
How national and/or regional ABS measures could be considered inconsistent with, or duplicative of, the PABS Annex
Time limitations and/or reporting requirements for Parties to share the outcomes of their review and any non-application of measures, taking into account all elements of the PABS System coming into operation simultaneously
Other arrangements for identification of contrary, inconsistent or duplicative measures
Applicable international, national and/or domestic law: The PABS System shall be “implement[ed] consistent with applicable international law and with applicable national and/or domestic law, regulations and standards related to risk assessment, biosafety, biosecurity and export control of pathogens, and data protection” [12.5(e)]
- Consider and/or address:
Applicable laws, regulations and standards and how they apply to PABS Materials and Sequence Information (e.g., to ensure efficient and safe exchange of pathogen material)
Provisions that need to be included in the PABS Annex to ensure consistency with applicable laws, regulations and standards
5. Governance and general provisions
Administration and coordination of the PABS System: “The PABS [Annex] shall define the terms for the administration and coordination of the PABS System by the [WHO]” [12.2], and in “coordination and operation of the PABS System, the [WHO] shall collaborate with relevant international organizations and relevant stakeholders” [12.2]
- Consider and/or address:
Provisions regarding the WHO Secretariat’s responsibilities, terms of administration, and operational dimensions including how the PABS System will be situated within the WHO Secretariat, and resourcing considerations compatible with WHO’s reprioritization efforts
Provisions to ensure complementarity and non-duplication with the administration and coordination of the PIP Framework, the Cali Fund and other relevant ABS instruments
Provisions regarding the development, review and amendment of terms of reference with laboratories and databases that are in line with the principles of the PABS System
Provisions regarding the development, review and amendment of terms of reference between the WHO and relevant international organizations and relevant stakeholders, including forms for collaboration, and the relevant international organizations and relevant stakeholders that should be involved based on their respective mandates, interests and capacities
The role of the Conference of the Parties (COP) and monitoring and review of the PABS System
- Consider and/or address:
Provisions regarding COP’s role in providing oversight, the Secretariat’s role in reporting to the COP (linked to Article 22), and mechanisms for monitoring and review to ensure the PABS System’s good functioning (e.g., access, contracts being negotiated and signed, vaccines, therapeutics and diagnostics distributed, and other benefits shared, as well as overall assessment of the System’s performance), including frequency and scope
Entry into operation: “All elements of the PABS System shall come into operation simultaneously in accordance with the terms of the PABS [Annex]” [12.2]
- Consider and/or address:
How all elements of the PABS System come into operation simultaneously and if any criteria or clarifications regarding operationalization are required
BANGLADESH
(Added on August 26, 2025)
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Bangladesh.pdf
BANGLADESH PABS TEXT PROPOSAL
OUTLINE & KEY ELEMENTS FOR ANNEX ON WHO PATHOGEN ACCESS AND BENEFIT SHARING SYSTEM (PABS SYSTEM)
This interim submission is intended to advance the PABS Annex negotiations at IGWG. This submission, which contains some suggested elements to be addressed by the Annex, is without prejudice to any further/revised elements and textual proposals and suggestions to be put forward jointly or individually as the discussion progresses.
A. OBJECTIVE
The objective of this Annex is to improve pandemic preparedness and response and strengthen the protection against the PHEIC and/or the Pandemic by establishing a safe, transparent and accountable multilateral WHO Pathogen Access and Benefit Sharing System pursuant to Article 12 of the Pandemic Agreement for, on an equal footing,:
(i) The sharing of PABS Materials and Sequence Information; and,
(ii) The access to vaccines, therapeutics and diagnostics (VTDs) and sharing of other benefits.
B. SCOPE
1. The Annex applies to the rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information.
2. For the purpose of the Annex “pathogen with pandemic potential” means [placeholder]
3. The PABS System shall operate complementarily to the PIP Framework. In recognition of their complementary nature, the operation of the PABS System shall extend to H5N1 and other influenza viruses with human pandemic potential, where cooperation under the PIP Framework is absent or insufficient.
[JUSTIFICATION: The two instruments have difference in their legal status. The difference in legal status doesn’t prevent the PABS System and the PIP Framework from being complementary, but it does create a hierarchy of enforceability. The proposed text has not taken into consideration this hierarchy of enforceability. The PIP Framework is not legally binding for the WHO Member states. The SMTA 2 under the PIP Framework between the WHO and the recipient entities is legally binding, which does not entail any legal obligations for the states. What if a state does not comply with the commitments made under the PIP Framework? We think that there should be provision for bringing states under obligations to make sure that they do cooperate during a PHEIC and/or Pandemic caused by influenza viruses with pandemic potential voluntarily and primarily through the PIP Framework and, if not, mandatorily through the PABS System.]
4. All elements of the PABS System shall come into operation simultaneously.
5. The PABS System shall operate regardless of origin or source of the PHEIC and/or Pandemic for realization of the purpose of the Pandemic Agreement.
[JUSTIFICATION: Unlike the IHR, 2005, the Pandemic Agreement may suffer from a legal loophole during a public health emergency, since it has not explicitly referred to its source neutrality in the texts. Source neutrality means that the treaty obligations under international law apply regardless of the origin of a public health threat—whether it arises from natural, accidental release, or deliberate use of pathogens (e.g., bioterrorism or bioweapon as a means of warfare). The Pandemic Agreement is implicitly source-neutral, as it focuses on pandemic prevention and response mechanisms, without distinguishing among the origins (by remaining silent on them). The PABS Annex should not follow the same approach of implicit source neutrality. Since, in the Pandemic Agreement, this is an absence rather than an explicit affirmation (of source neutrality), it remains an issue subject to interpretation rather than a formally stated principle. In legal interpretation, it is highly likely to suffer from the 'scope limitation by silence'. Since article 4 of the Pandemic Agreement has addressed only natural causes, the rules of treaty interpretation (Vienna Convention on the Law of Treaties article 31) may favour narrow interpretation (i.e., applicable to natural outbreaks only) of the scope of the Pandemic Agreement and the PABS System during a public health emergency.
Given the formidable challenge of source attribution, and its vulnerability to geo-political manoeuvring (e.g., even a naturally occurring Pandemic may be labelled as one originating from lab leak or deliberate use of pathogens), the Pandemic Agreement and the PABS System may struggle to deliver in such scenario (further details can be shared in support of this claim). Arguments may be made to address the outbreak under the Biological and Toxin Weapons Convention (BTWC) and/or UN Secretary General’s Mechanism (UNSGM). Countries may argue for making pandemic response conditional to attribution, accountability, and enforcement, which BTWC cannot do because of its lack of verification, and compliance mechanism. This is a grave danger facing public health emergency response, which the drafters of International Health Regulations (IHR) duly sensed. Accordingly, they carefully avoided implicit source neutrality. Instead, they adopted explicit source neutrality (IHR article 7) by clearly mentioning 'regardless of origin or source'. To our understanding, explicit source neutrality approach is, undoubtedly, the potent way to safeguard any PHEIC and/or Pandemic response from geo-political manoeuvring, as numerously manifested through IHR's real-time efficacy. We think that the PABS System should adopt similar approach to secure itself against any unnecessary debate over its scope of application. Including this sub-para 5 into the Annex can also make sure that the Pandemic Agreement has clear mandate over any PHEIC and/or Pandemic irrespective of source or origin.]
C. DEFINITIONS FOR THE PURPOSE OF THE ANNEX
1. PABS Materials: (i) isolated wild-type pathogens assessed to have pandemic potential, and parts thereof; (ii) any modified forms of such pathogens; and (iii) any other materials derived from, generated or prepared using the materials described in (i) and (ii).
2. PABS Sequence Information: any data or information generated from PABS Materials through the application of sequencing technologies
3. Authorized national laboratories: laboratories authorized and designated by a Party to provide PABS Materials and/or PABS Sequence Information to the PABS System and recognized as part of the WHO Coordinated Laboratory Network.
4. Clinical Specimens: materials taken from humans including specimens collected from the respiratory tract (for example, swabs and aspirated fluid), and also blood, serum, plasma, faeces, and tissues.
5. Originating laboratory: a national authorised laboratory that sent the initial clinical specimens and/or the isolated pathogen with pandemic potential
6. Participating Manufacturer: public and/or private entities that develop and/or manufacture vaccines, therapeutics and diagnostics for pathogens with pandemic potential including, but not limited to, academic institutions, government owned or government subsidized entities, nonprofit organizations or commercial entities.
7. WHO Coordinated Laboratory Network an international network of authorized national laboratories coordinated by WHO and governed by the PABS System under the supervision
of COP. To be a part of the WHO Coordinated Laboratory Network, an authorized national laboratory meets the criteria for designation and agree to comply with terms and conditions as set out in the Standard Contract 1, the applicable Terms of Reference, and any other requirements of the PABS system.
8. [placeholder: affordable prices]
(the above list below is non-exhaustive and may be expanded)
D. FRAMEWORK OF THE PABS SYSTEM
1. The PABS System & rapid and timely access to PABS Materials and Sequence Information
1.1 PABS Materials & Traceability Measures
1. 1.1 WHO Coordinated Laboratory Network (WCLN)
a. WCLN coordinated by WHO under the oversight of COP, shall be established. The network shall link authorised national laboratories that meet the criteria for participating in the WCLN and agree to accept the Terms of Reference (ToR), the legally binding terms and conditions applicable to the sharing/utilization of PABS Materials and PABS Sequence Information, as well as other requirements of the PABS System.
b. When a Party has access to a pathogen with pandemic potential identified from within a territory under its jurisdiction, it shall, taking into account national risk assessment and applicable biosafety, biosecurity and data protection standards, notify the PABS Tracking Mechanism (PABS TM) established as per para 4.1 (2) of this Annex through authorized national laboratories and obtain a PABS electronic label prior to providing the materials to one or more laboratories, of its choice, participating in WCLN.
c. National laboratories shall make, as feasible, efforts to ensure that PABS biological materials that they provide to WCLN contain viable material and are accompanied by information as agreed in the PABS Tracking Mechanism (PABS TM) and other clinical and epidemiological information needed for risk assessment.
d. WHO shall be responsible for facilitating the funds required to cover the costs related to shipment of PABS Materials to and within the WHO Coordinated Laboratory Network, by developing country Parties.
e. The Parties may also provide PABS biological materials directly to any other party or body on a bilateral basis provided that the same materials are provided on a priority basis to WCLN under this Annex.
[JUSTIFICATION: States Parties to the Nagoya Protocol can share the biological materials with other parties based on bilateral contracts.]
1.1.2 Standard Legally Binding Contracts Applicable to the Sharing of PABS Materials and PABS Sequence Information
a. All transfers of PABS Material by an authorised national laboratory to a laboratory that is part of WCLN and further transfers within WCLN are subject to Standard Contract 1.
b. By providing PABS Materials to laboratories designated as part of the WHO Coordinated Laboratory Network, the provider Party provides their consent for the onward transfer to and use of PABS Materials by Recipient Entities outside the WCLN that have signed Standard Contract 2 with WHO. Recipients agree not to transfer or share PABS materials to entities outside WCLN that have not signed Standard Contract 2.
c. Both Standard Contract 1 and 2 shall contain terms and conditions applicable to the storage, sharing, and use of sequence Information generated by recipients from PABS Materials.
1.2 PABS Sequence Information & Traceability Measures
1.2.1 WHO Coordinated Database Network (WCDN)
a. Sharing of any PABS sequence information shall be through a WHO PABS Sequence Database that is transparent and accountable to Parties and implements requirements set out in sub-paragraph (f) and COP decisions. WHO may host such a database or may, following a COP decision, organise an existing database to host the WHO PABS Sequence Database.
b. Other databases may access and share PABS sequence information, subject to the database entering into a legally binding contract with WHO, agreeing to comply with terms and conditions set out in sub-paragraph (f) (hereinafter referred to as “Participating Databases”).
c. WHO PABS sequence database, along with participating databases constitute WCDN.
d. The Parties shall notify the PABS Tracking Mechanism (PABS TM) and obtain a PABS electronic label prior to providing the Sequence Information to WCDN.
e. In the event that the publication of Sequence Information has been considered sensitive by the Party providing the PABS Biological Materials and Sequence Information in some instances, the WHO Director General shall consult with the PABS Advisory Group established as per para 5.2 of the Annex on the best process for further discussion and resolution of issues relating to the handling of Sequence Information under this annex.
f. WHO PABS Sequence Database and other Participating Databases hosting PABS Sequence
Information to comply with terms and conditions including:
(i) require registration and acceptance of Data Access Agreement (Standard Contract 3) from all users of database intending to access PABS Sequence Information;
(ii) provide access to PABS Sequence Information to all registered users, whose identity is verified, without any fee charged and without any form of discrimination.
(iii) attach persistent unique labels to sequence files or datasets in a manner identifying originating country of the PABS materials from which sequence information is generated
(iv) make available facilities for registered users to link their scientific or other publications, provide information of R&D progress and R&D outcomes that use/refer to PABS Sequence Information;
(v) provide information related to usage of databases, including a list of registered users as well as other particulars, to WHO periodically and/or upon request.
(vi) agree to comply with all requirements of PABS system and act in accordance with WHO/COP decisions in this regard.
(vii) In the event, the participating database is generating revenue from the sharing/utilization of PABS Sequence Information, the participating database agrees to contribute towards monetary benefit-sharing;
1.2.2 Standard Legally Binding Contract Applicable to the Sharing of PABS Sequence Information
a. Parties to take measures to ensure that PABS sequence information is shared through the WHO PABS Sequence Database and consistently with its requirements.
b. Laboratories within WCLN to share all PABS Sequence Information through the WHO PABS Sequence Database.
c. Submission of PABS Sequence Information to be based on the format as decided by the COP, and such format shall include requiring the submitter to provide information on the country of the originating laboratory
2. The Parties agree that the users shall not claim any intellectual property or other rights that limit the access or use of the biological materials shared, their genetic parts or components, including genetic sequence data or parts thereof.
3. Fair and Equitable Benefit-Sharing from Recipients of PABS Materials and Sequence Information (to be reflected in Standard Contracts 2 and 3)
3.1 Annual Monetary Contributions (applicable to all recipients accessing PABS Materials and/or PABS Sequence Information)
• When revenue is generated from the sharing/utilization of PABS Materials and/or PABS Sequence Information, to annually contribute x% of total annual revenue for each product or service developed and commercialised using the PABS System. Annual revenue includes all financial benefits such as income from sales and royalties.
3.2 Non-monetary Benefit Sharing
a. Participating manufacturer:
(i) in the event of a pandemic emergency, to make available make available to the WHO rapid access targeting 20% of their real time production of safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency, provided that a minimum threshold of 10% of their real time production is made available to the WHO as a donation, and the remaining percentage, with flexibility based on the nature and capacity of each participating manufacturer, is reserved at affordable prices to the WHO, in accordance with paragraph 6 of Article 12.
(ii) to provide to WHO on request, at not-for-profit prices and on a priority basis, the vaccines, therapeutics and diagnostics, for stockpiling and/or to supply affected developing countries, to prevent, prepare for and respond to a PHEIC. The set-asides to be determined in IGWG
(iii) in the event of a PHEIC and/or a pandemic emergency, to grant WHO non-exclusive licenses that can be sublicensed to manufacturers in developing countries, especially to those that provided PABS Materials and PABS Sequence Information, for the development and/or production of pandemic-related products needed in developing countries, to expand supply rapidly, ensuring prompt equitable access in developing countries. Such a license to include provision of the full regulatory dossier, technical know-how, and any necessary materials (e.g. cell-lines etc.).
b. Recipients accessing PABS Materials and/or PABS Sequence Information for strictly for Non-Commercial uses (not intended to generate revenue)
1. The Parties agree that entities that use PABS Materials and Sequence Information for non-commercial purposes shall:
(a) include scientists from originating laboratories, institutions or countries in research collaboration and scientific publications;
(b) acknowledge the providers of the PABS Materials and Sequence Information in relevant presentations or publications;
(c) contribute to public dissemination and transparency of publication or any other outputs by making the results available through the PABS TM with an additional obligation of reporting through the WHO PABS Sequence Database such that it shall be linked to the sequence information accessed;
(d) actively engage in scientific or academic collaborations, training and capacity building activities, and consider voluntary monetary contributions to support the PABS System;
2. In the event that a non-commercial user becomes a commercial user or a relevant manufacturer or entities for the purposes of commercial use and/or utilization under this section, that user shall be subject to terms and conditions applicable to commercial users and relevant manufacturers and entities.
c. Other Recipients
[placeholder to add further non-monetary benefit sharing as discussion progresses]
3.3 Other Non-monetary Benefit Sharing
[Note: These following paragraphs, which detail responsibilities of both the Parties and the Entities can be either voluntary or mandatory or combined depending on the bargaining at the negotiations at IGWG. Accordingly, the texts of the articles will develop.]
a. Capacity building & technical assistance
(i) The Parties shall cooperate in the capacity-building, capacity development and strengthening of human resources and institutional capacities to effectively implement this PABS System in developing country Parties including through existing global, regional, subregional and national institutions and organizations.
(ii) The need of developing country Parties shall be taken fully into account for capacity-building and development to implement this Annex.
(iii) The WHO shall play a pioneering role in the capacity-building, capacity development and strengthening of human resources and institutional capacities to effectively implement this PABS system in developing country Parties particularly to make sure that the national laboratories can meet the criteria to participate in the WCLN.
(iv) Upon request, the Parties with advanced laboratory capacities shall work with WHO andother Parties, particularly developing countries, to develop national laboratory and pathogen surveillance capacity, including:
(a) to conduct early detection, isolation and characterization of pathogens with pandemic potential;
(b) to participate in pandemic risk assessment and response;
(c) to develop research capacity related to pathogens with pandemic potential;
(v) Upon request, the Parties with advanced regulatory capacity shall work with WHO, particularly in developing countries, to strengthen the capacity of regulatory authorities to carry out the necessary measures for the rapid approval of safe and effective VTDs, including products developed from the use of the PABS Materials and Sequence Information.
(vi) The Parties shall make publicly available information on the notification of health regulatory approval of VTDs for pathogens with pandemic potential including those developed from the use of the PABS Materials and Sequence Information.
(vii) [Capacity building obligations of the actors other than the Parties may be included in the standard contract texts.]
b. Research and development cooperation
(i) The Parties shall cooperate in scientific research and development involving the PABS Materials and Sequence Information sourced from developing countries.
(ii) The Parties shall ensure that the benefits arising from such research are shared in a fair and equitable manner, including but not limited to: sharing research results, joint authorship in publications, co-ownership of intellectual property rights, royalties from commercial products and access to scientific knowledge, data and relevant technologies.
(iii) Acknowledging that capacity building in scientific and technological fields shall be a core component of such cooperation with the aim of empowering developing countries to participate fully in the research process and the subsequent utilization of its results, the Parties with advanced research and development capabilities shall encourage their commercial and non- commercial users of the PABS Materials and Sequence Information to include originating laboratories, institutions or countries in research and development collaboration.
(iv) [R&D obligations of the actors other than the Parties may be included in the standard contract texts.]
c. Technology transfer
(i) The WHO Director General shall work closely with the Parties and VTD manufacturers to increase VTD supply, including its strategies to build new production facilities particularly in developing countries and through transfer of technology, skills and know-how.
(ii) The Parties shall encourage VTD manufacturers to make specific efforts to transfer these technologies to other countries, particularly developing countries, as appropriate.
(iii) Technology transfer shall be conducted in a manner consistent with applicable national laws and international laws and obligations facilitated progressively over time, on mutually agreed terms, and be suitable to the capacity of recipient Parties, to empower developing countries to study and manufacture VTDs.
(iv) [Technology transfer obligations of the actors other than the Parties may be included in the standard contract texts.]
d. [Non-exhaustive list of other non-monetary benefits can be mentioned following the Nagoya Protocol]
4. Other key provisions administering the PABS System
Tracking Mechanism (PABS TM) to record all transfers of PABS
4.1 Transparency, Traceability & Reporting Mechanism
1. Access to PABS Materials and Sequence Information shall be subject to the prior informed consent of the Party providing such resources and mutually agreed terms between providers and users of such resources.
2. WHO to establish a PABS Materials
3. WHO to make publicly available:
a. the list of registered recipients of PABS Sequence Information;
b. the list of WCLN labs
c. monetary contributions received from each recipient and their utilization;
d. standard contracts 2 & 3 concluded with each recipient.
4.2 Consistency with applicable practices, rules and laws related to risk assessment,
biosafety, biosecurity, export control of pathogens, and data protections
1. The Parties shall utilize the PABS system in their pandemic surveillance, risk assessment and response, early warning information and services to all the Parties. The use of PABS Materials under the system shall follow applicable practices, rules and laws related to risk assessment and response.
2. The sharing of the PABS materials shall be subject to appropriate biosafety guidelines and regulations and employ laboratory protection best practices.
3. Diversion of the PABS Materials and Sequence Information and their derivatives for military purpose is prohibited and shall be in violation of the applicable international norms and law.
[JUSTIFICATION: Given the sensitivity and importance of the issue, it should be mentioned in the general text of the Annex in addition to the Standard Contracts. The benefit of placing it here is that the obligations will cover all including the Parties, WCLN laboratories, WCDN databases and other users of PABS Materials and Sequence Information.]
4. The Parties shall harmonise their national legislation and practices on export control with the requirements of the PABS system.
5. When the PABS Materials and Sequence Information are accessed, the individuals or entities accessing those make sure that sensitive information is protected and only shared appropriately.
The PABS system demands due diligence from users, who must seek, keep, and transfer relevant information, and compliance measures to prevent unauthorised access or use.
4.3 Relationship with other international frameworks, regulations and agreements
1. The PABS System shall consistently operate with the objectives of the Nagoya Protocol of the Convention on Biological Diversity.
2. The PABS System shall operate complementarily to the PIP Framework. WHO shall continue to apply the PIP Framework to the sharing of H5N1 and other influenza viruses of human pandemic potential, including benefit sharing. In recognition of their complementary nature, the operation of the PABS System shall extend to H5N1 and other influenza viruses with human pandemic potential, where cooperation under the PIP Framework is absent or insufficient.
[Note: Part of it is also mentioned in SCOPE of the Annex.]
3. [The relationship of the PABS system with the International Health Regulations needs to be mentioned.]
4. The PABS system shall operate without prejudice to and complement the relevant provisions of the 1972 Biological and Toxin Weapons Convention (BTWC).
[JUSTIFICATION: Discussions under the BTWC are ongoing to facilitate cooperation and assistance through mechanisms in the fields of biological science and technology under its
Article X. The Convention has also envisaged international response to use of deliberate use of pathogens as mentioned in Article VII.]
4.4 Alignment of national legislation or regulatory requirements on access and benefit sharing and dispute resolution
1. The Parties shall take all steps to facilitate the rapid and timely manufacture and export of vaccines, therapeutics and diagnostics, and to operationalise other benefit-sharing obligations that recipients have committed to provide to prevent, prepare for and to respond to a PHEIC and/or a pandemic emergency for pathogens covered by the PABS system.
2. Each Party, in respect of such a user operating within its jurisdiction, shall take the necessary legislative, administrative or policy measures to ensure non-state actors with all components of the PABS System comply with the terms and conditions of the PABS System.
3. The Parties shall cooperate and take appropriate measures, such as conditions in public procurements or on public financing of research and development, pre-purchase agreements, or regulatory procedures, to ensure that users of the PABS System comply with their respective benefit sharing commitments, including that relevant manufacturers and entities conclude the PABS contracts as early as possible.
4. The Parties shall take appropriate, effective and proportionate measures to address situations of non-compliance with measures adopted in accordance with paragraph 1 and 2 above.
5. The Parties shall, as far as practicable and as appropriate, cooperate in cases of alleged violation of domestic access and benefit sharing legislation or regulatory requirements referred to in paragraph 1 and 2 above.
6. The Parties may bring to the attention of the WHO Director General allegations of non-compliance by WCLN laboratories, WCDN databases and any other recipients with their respective terms of reference or the Standard Contracts for further necessary actions elaborated at para 5.4, 5.5. and 5.6.
5. Governance, Monitoring and Review of the PABS System
5.1 PABS system to be coordinated and administered by the WHO, under authority of the COP. WHO to take all measures necessary to ensure a fair, transparent, equitable, effective, efficient and accountable implementation of the PABS System.
5.2 An independent, free of conflict of interests, oversight “PABS Advisory Group” to be established to monitor and to provide guidance to WHO on the functioning of the PABS system in accordance with the terms of reference for the Advisory Group. The PABS Advisory Group to present an annual report to the COP on its evaluation of the implementation of the PABS system, with its recommendations for COP’s approval.
5.3 The Director-General, in consultation with Member States, to ensure that the PABS Advisory Group is based on fair and equitable representation of the WHO regions, taking into account the importance of representation from developing countries. The PABS Advisory Group shall comprise xx members drawn from each WHO Region, with a skill mix of internationally recognized policy makers, public health experts and technical experts.
5.4 In the event of any alleged breaches of the terms of reference or the Standard Contract by a WCLN laboratory, the Director-General shall review the circumstances and discuss with the PABS Advisory Group any appropriate action in response to those breaches. Where there has been a serious breach, the Director-General may consider suspending or revoking the WHO designation of the relevant laboratory.
5.5 In the event of any alleged breaches of the terms of reference or the Standard Contract by WHO PABS Sequence Database or participating databases, the Director-General shall review the circumstances and discuss with the PABS Advisory Group any appropriate action in response to those breaches. Where there has been a serious breach, the Director-General may consider suspending or revoking the WHO designation of the relevant database.
5.6 In the event of any alleged breaches of the Standard Contract by receiving entities (outside the WCLN laboratory, WHO PABS Sequence Database, and participating database), the Director-General shall review the circumstances and discuss with the PABS Advisory Group any appropriate action in response to those breaches. Where there has been a serious breach, the Director-General may consider suspending or revoking the access of such receiving entities.
5.7 Monitoring of the PABS System
[Note: There should be provision on monitoring on the implementation of the PABS System.
The WHO Director-General may be tasked with reporting to the COP covering several issues. PIP Framework monitoring provision can be consulted.]
5.8 Review of the PABS System
[Note: There should be provision of review by COP of the PABS System to check if the system is functioning and needs further improvement.]
E. STANDARD LEGALLY BINDING CONTRACTS STANDARD CONTRACT 1:
TERMS AND CONDITIONS APPLICABLE TO NATIONAL AUTHORISED LABORATORIES AND WCLN LABORATORIES
Legally binding contract between the Provider (national authorised laboratory sending PABS Materials and Sequence Information) and Recipient (WCLN laboratory receiving PABS Materials and Sequence Information), to be concluded when becoming a member of WCLN. Terms and conditions to be included in the standard contract include:
1. The recipient agrees to comply with its ToR and to use the PABS Material and clinical specimen solely for public health purposes as set out in the Terms of Reference, for the prevention, preparedness and response to a pandemic, and not-for-profit.
2. All transfers of PABS Materials to be recorded in the PABS Materials Tracking Mechanism.
3. Onward transfer and use of the PABS Materials to all WCLN laboratories to be on the same terms and conditions applicable to WCLN; and to entities outside the WCLN subject to the Entity concluding Standard Contract 2 with the WHO.
4. Recipient to actively seek participation of scientists from originating laboratories, especially those from developing countries, in scientific or R&D projects associated with research on PABS Material and Sequence Information from their countries, as well as actively engage them in preparation of manuscripts for presentation, publication or any other outcomes;
5. Recipient to appropriately acknowledge in presentations and publications the contributions of collaborators, including originating laboratories/countries providing the PABS Material and Sequence Information.
6. Intellectual property shall not be sought or asserted over any PABS Materials and Sequence Information, or parts thereof, in any form, including any modified form or for any use.
7. The recipient to regularly and/or on request, share all analyses and outcomes from the sharing/utilization of PABS Materials and PABS Sequence Information with the Provider.
8. Sharing of PABS sequence information to be through the WHO PABS Sequence Database and agree to comply with the terms and conditions as applicable to the users of PABS Sequence Information.
9. Agrees to comply with COP decisions with respect to the PABS system
Criteria for an authorized national laboratory to ensure biosafety and biosecurity.
STANDARD CONTRACT 2:
TERMS AND CONDITIONS APPLICABLE BETWEEN WHO AND ENTITIESOUTSIDE OF WCLN
Legally binding contract between: WHO and the Recipient Entity (outside of the WCLN laboratory receiving PABS Materials and PABS Sequence Information). The recipient entity can access PABS Materials and PABS Sequence Information from WCLN, subject to the conclusion of a legally binding contract with WHO. Terms and conditions of the standard contract include:
1. using the PABS Materials and PABS Sequence Information solely and strictly for research and development in connection with pathogens with pandemic potential within the scope of the PABS system for the prevention, preparedness and response to a pandemic.
2. not be used in any activity that may lead to the development, or production of biological agents, toxins, weapons, equipment, or means of delivery specified in Biological Weapons Convention;
3. to keep WHO informed of all uses of PABS Materials and PABS Sequence Information, as well as any changes to their intended use. Any use of PABS Materials and Sequence Information outside the scope of the PABS System must obtain prior informed consent from the originating country.
4. transferring the PABS Materials only if the prospective recipient has concluded the required contract with WHO. Any such transfer shall be reported to the WHO through the PABS Tracking Mechanism.
5. sequence information generated by the Recipient from the PABS Materials, sharing/transfer through WHO PABS Sequence Database and recipient to comply with terms of data access agreement (standard contract 3)
6. not to seek or assert intellectual property over any PABS Materials and PABS Sequence Information, or parts thereof, in any form, including any modified form or for any use.
7. to comply with monetary and non-monetary benefit-sharing obligations
8. Agrees to comply with COP decisions with respect to the PABS system
STANDARD CONTRACT 3: DATA ACCESS AGREEMENT/ TERMS AND CONDITIONS APPLICABLE TO ANY PERSON/ENTITY ACCESSING PABS SEQUENCE INFORMATION FROM WHO PABS SEQUENCE DATABASE/PARTICIPATING DATABASES
Legally binding contract for parties - the Database, WHO and person/entity seeking access to PABS Sequence Information. Access to PABS Sequence Information is subject to registration and acceptance of data access agreement with terms that include:
1. use solely and strictly for research and development in connection with pathogens with pandemic potential within the scope of the PABS system for the prevention, preparedness and response to a pandemic;
2. not be used in any activity that may lead to the development, or production of biological agents, toxins, weapons, equipment, or means of delivery specified in Biological Weapons Convention;
3. transfer or share sequence information only if the prospective recipient is also a registered user either of the WHO PABS Sequence Database or a Participating Database;
4. acknowledge the contributions of the persons and institutions who submitted the sequence information as well as the originating laboratories that first collected the clinical specimen and isolated the pathogen with pandemic potential;
5. actively seek the participation of scientists from originating laboratories/countries of PABS Materials and clinical specimens from which the PABS Sequence Information was generated, especially those from developing countries, in scientific or R&D projects associated with the PABS Sequence Information, and to actively engage them in the development and implementation of the projects;
6. not seek or assert intellectual property over PABS Sequence Information or parts thereof, in any form, including any modified forms or for any use;
7. Agrees to comply with the monetary and non-monetary benefit-sharing obligations.
8. Agrees to comply with COP decisions with respect to the PABS system
(Standard contract 3 may potentially be in a form of a click-wrap agreement)
BRAZIL
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Brazil.pdf
MISSION PERMANENTE DU BRESIL AUPRES DE L'OFFICE DES NATIONS UNIES ETDES AUTRES ORGANISATIONS INTERNATIONALES A GENEVE
Chemin Camille-Vidart 15, 1202 Genève
N° 3802025
The Permanent Mission of Brazil to the United Nations Office and other International Organizations in Geneva presents its compliments to the World Health Organization and has the honour to submit, as an attachment, Brazil's text proposals, with elements to be addressed by the Annex of the WHO Pandemic Agreement.
The Permanent Mission of Brazil avails itself of this opportunity to renew to the World Health Organization the assurances of its highest consideration.
CÃODOBRA URDEN PRОО GENEBR
Geneva, August 14, 2025
To
World Health Organization Geneva
GENERAL PROPOSAL AND OUTLINE SUBMITTED BY BRAZIL TO THE PABS ANNEX PURSUANT TO ARTICLE 12 OF THE PANDEMIC AGREEMENT, AS APPROVED BY RESOLUTION WHA78.1
Pathogen Access and Benefit-Sharing System (PABS) Annex
Recalling Resolution WHA78.1, which adopted, pursuant to Article 19 of the Constitution of the World Health Organization, the WHO Pandemic Agreement and decided to establish an openended Intergovernmental Working Group (IGWG);
Emphasizing the role of the International Health Regulations (2005), adopted through resolution WHA58.3 (2005), and subsequently amended through resolutions WHА67.13 (2014), WHA75.12 (2022), and WHA77.17 (2024), in pandemic prevention, preparedness and response, and the need for coherence and complementarity in the implementation of the International Health Regulations (2005) and the WHO Pandemic Agreement;
Recognizing the continuing threat of pathogens with pandemic potential which calls for enhancing pandemic prevention, preparedness and response and for refraining from taking measures that adversely affect it;
Reaffirming that States have sovereign rights over their own biological resources, as well as the sovereign right to legislate and implement laws, including national access and benefit-sharing legislation;
Recalling the importance of ensuring access to human pathogens for public health preparedness and response purposes,
Considering Article 4.4 of the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits arising from their utilization to the Convention on Biological Diversity, which states that where a specialized international access and benefit-sharing instrument applies that is consistent with, and does not run counter to the objectives of the Convention and this Protocol, this Protocol does not apply for the Party or Parties to the specialized instrument in respect of the specific genetic resource covered by and for the purpose of the specialized instrument,
Bearing in mind the parameters established by Article 12 and other applicable provisions of the Pandemic Agreement, Have agreed as follows:
ARTICLE 1: OBJECTIVE
This Annex is developed pursuant to Article 12 of the Pandemic Agreement and establishes the modalities for the implementation of the multilateral system for access and benefit sharing of pathogen materials and sequence information ("PABS System"), in order to render functional a fair, transparent, and accountable multilateral system for the sharing of pathogens and sequence information. The system aims to facilitate rapid access, equitable sharing, and benefit sharing related to pandemic pathogens.
ARTICLE 2: SCOРЕ
Paragraph 1: This annex applies to the timely and equitable sharing of "Materials and Sequence Information on Pathogens with Pandemic Potential" (hereinafter referred to as "PABS Materials and Sequence Information"). It also covers the sharing of benefits derived from the use or sharing of such materials and information.
Paragraph 2: For purposes of this annex, "pathogen with pandemic potential" shall be defined as any pathogen that has been identified to infect a human and that is: novel (not yet characterized) or known (including a variant of a known pathogen), potentially highly transmissible and/or highly virulent with the potential to cause a public health emergency of international concern that may develop into a pandemic emergency; in addition to the high potential for transmissibility and severity, it can also be taken into consideration the capacity for severe socioeconomic impact, overloading health systems, and disproportionately affecting vulnerable populations. The WHO Secretariat will make available non-exhaustive list of pathogens with pandemic potential based on its global pathogen prioritization process, which can serve as an initial indication.
Paragraph 3: The PABS System shall operate in complementarity with the Pandemic Influenza Preparedness (PIP) Framework, and shall not, hence, regulate the sharing of influenza virus material or sequence information.
Paragraph 4: All elements of the PABS System shall enter into force simultaneously.
ARTICLE 3: DEFINITIONS
Paragraph 1: PABS Materials and Sequence Information: includes data, metadata or information derived from biological or genetic materials. This definition is operational and may be revised by the Conference of the Parties of this instrument, taking into account changing circumstances.
[OR SEPARATE MATERIALS FROM SEQUENCE INFORMATION]
PABS Materials: isolated wild-type pathogens with pandemic potential, and parts thereof, modified pathogens, and any other materials derived from, generated or prepared using the PABS Materials.
PABS Sequence Information: any data or information generated from PABS Materials through the application of sequencing technologies
Paragraph 3: "Authorized National Laboratories" are laboratories authorized and designated by a Party, recognized within the WHO Coordinated Laboratory Network, to provide PABS Materials and Sequence Information.
Paragraph 4: "Clinical Specimens" include samples obtained from humans, such as respiratory specimens (e.g., swabs, aspirates), blood, serum, plasma, feces, and tissues.
Paragraph 5: "Originating Laboratory" is the authorized national laboratory that first sent the clinical specimen or the isolated pathogen with pandemic potential.
Paragraph 6: "Participating Manufacturer" refers to public or private entities, including academic institutions, government-owned [or government subsidized entities], or nonprofit organizations, involved in developing or manufacturing vaccines, therapeutics, and diagnostics for pathogens with pandemic potential.
Paragraph 7: WHO Coordinated Laboratory Network is an international network of authorized laboratories managed by WHO, governed by the PABS System under the supervision of the Conference of the Parties (COP). Membership requires meeting designated criteria and compliance with contractual terms.
Paragraph 8: "Pandemic related health products" means those relevant health products that may be needed for prevention, preparedness and response to pandemic emergencies (from HIR)
ARTICLE 4: SYSTEM STRUCTURE
Section 4.1: WHO Coordinated Laboratory Network (WCLN)
Paragraph 1: The WCLN will be established under WHO coordination, linking authorized national laboratories that meet the criteria and agree to abide by the Terms of Reference (ToR), which are legally binding.
Paragraph 2: WHO shall facilitate the funding required for transportation of PABS Materials among laboratories within the network, supported by developing country Parties.
Section 4.2: Legally Binding Contracts
Paragraph 1: All transfers of PABS Materials within the network are subject to Standard Contract 1, which establishes the terms and conditions for sharing.
Paragraph 2: By providing Materials, Parties consent to onward sharing under Standard Contract 2, restricting transfers outside the network unless formal agreements are in place.
Paragraph 3: Both contracts include terms on storage, sharing, and use of Sequence Information generated from Materials.
Section 4.3: Sequence Information & Traceability
Paragraph 1: Sharing of Sequence Information occurs via a WHO-managed database, which may host or coordinate with existing databases, ensuring transparency and accountability.
Paragraph 2: Access to the Sequence Database and Participating Databases requires registration, adherence to data access agreements, and verification of user identity.
Paragraph 3: Sequence data must be labeled with identifiers linking the information to the originating country.
Paragraph 4: Users must report their analyses and publications to WHO, and agree to terms ensuring compliance and benefit-sharing, including contributions from revenue.
ARTICLE 5: BENEFIT SHARING
Paragraph 1: Access to PABS Materials and Sequence Information is contingent upon agreement to share benefits. Those benefits will be listed in the legally binding contracts as alternatives for different categories of users, and will include:
a) Annual financial contributions by commercial users based on revenues generated from pandemic-related health products developed using PABS Materials or Sequence Information;
b) Capacity-building and technical assistance;
c) Research and development cooperation;
d) Open and timely access to research data and results. Includes scientific findings, analyses, and outcomes derived from PABS use.
e) The granting of non-exclusive licenses to manufacturers in developing countries, for the effective production and delivery of vaccines, therapeutics and diagnostics; and
f) Other forms of transfer of technology as mutually agreed, including transfer of relevant knowledge, skills and technical expertise.
Paragraph 2: When revenue arises from commercialization of products which were developed thanks to the sharing or utilization of Materials or Sequence Information, participating manufacturers shall contribute a percentage of the income to benefit-sharing;
Paragraph 3: In the event of a pandemic emergency, each participating manufacturer shall make available to the World Health Organization, pursuant to legally binding contracts signed with the World Health Organization, rapid access targeting 20% of their real time production of safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency, provided that a minimum threshold of 10% of their real time production is made available to the World Health Organization as a donation, and the remaining percentage, with flexibility based on the nature and capacity of each participating manufacturer, is reserved at affordable prices to the World Health Organization.
ARTICLE 6: TRANSPARENCY, TRACEABILITY AND REPORTING
Paragraph 1: Access to PABS Materials and Sequence Information shall be subject to prior informed consent of the providing Party, and mutually agreed terms between providers and users.
Paragraph 2: WHO shall establish a PABS tracking mechanism to record all transfers of Materials and Sequence Information within the system.
Paragraph 3: WHO shall publicly disclose:
a) the list of registered recipients of Sequence Information;
b) the list of laboratories within the WHO Coordinated Laboratory Network (WCLN);
c) monetary contributions received from each recipient and their utilization;
d) the contracts (Standard Contracts 2 and 3) concluded with each recipient.
ARTICLE 7: GOVERNANCE OF THE SYSTEM
Paragraph 1: The PABS System shall be coordinated and administered by WHO, under the authority of the Conference of Parties (COP), ensuring fair, transparent, and effective implementation.
Paragraph 2: An independent, conflict-of-interest-free oversight "PABS Advisory Group" shall be established to monitor the system's functioning and provide guidance. The Group shall submit annual evaluations to the COР.
Paragraph 3: The Director-General, in consultation with Member States, shall ensure that the Advisory Group has balanced regional representation, including experts and stakeholders from developing countries.
Paragraph 4: In cases of breaches-by laboratories, databases, or recipients-the DirectorGeneral shall review and, if necessary, suspend or revoke recognition or designation.
Paragraph 5: Parties shall facilitate the rapid manufacture and export of vaccines, therapeutics, and diagnostics, and ensure compliance with benefit-sharing obligations.
ARTICLE 8: STANDARD LEGALLY BINDING CONTRACTS
Section 8.1: Contract 1 – Between the provider (national authorized laboratory) and WCLN recipient
a) The provider agrees to supply Materials and Information strictly for public health, non-profit purposes.
b) Transfers are recorded, and onward transfers only occur under Contract 2.
c) Recipients shall acknowledge origin and contributions, and agree not to claim intellectual property rights over Materials or Sequence Information.
d) All results and analyses must be reported back, and Sequence Information must be shared via the WHO database.
Section 8.2: Contract 2 - Between WHO and external entities outside WCLN
a) Use of Materials and Sequence Information is strictly for research related to pathogens with pandemic potential.
b) Use outside scope requires prior WHO approval.
c) Transfers must be reported and follow the agreed data formats.
d) No intellectual property claims; benefits from use must contribute to benefit-sharing obligations.
Section 8.3: Contract 3 - Data Access Agreement
a) Users agree to use Sequence Information solely for research in accordance with system rules.
b) Access is conditional on registration, signing the agreement, and compliance with confidentiality, rights acknowledgment, and benefit-sharing.
ARTICLE 9: DISPUTE RESOLUTIONS
Standard legally binding contracts in the PABS System shall contain dispute resolution clauses which determine that if a dispute cannot be resolved through negotiations or other non-binding means of the parties' choice, it shall be subject to binding arbitration on conditions that are mutually agreed by the parties.
CENTRAL AFRICAN REPUBLIC
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Central_African_Republic.pdf
The text below has been machine translated from the original French version.
Submission to the Intergovernmental Working Group (IGWG) for the Negotiations of the BSAP Annex Central African Republic
Submission from the Central African Republic
This submission is made in response to the IGWG's invitation to WHO Member States to submit initial text proposals, including elements to be included in the PABS Annex, to support the Bureau in developing a draft framework. We submit the following without prejudice to future text proposals.
Key Elements for the PABS Annex:
1. Benefit Sharing – VTD Stockpiles to Prevent PHEICs and Pandemics
A fundamental element of the PABS system is that benefit sharing must ensure equitable and guaranteed access to VTDs from the initial stages of outbreaks, to prevent their development into PHEICs, and during PHEICs to prevent pandemics. This guarantee should be materialized in the form of mandatory stockpiles for developing countries.
VTD manufacturers using PABS Materials and sequence information must commit to providing at least 15% of their production in real time (or from stockpiles in the absence of active production) to the WHO for these purposes, at least half of which will be free and the other half at cost.
This commitment should be applicable not only during emergency periods, but also during non-emergency periods, to build up WHO stockpiles and allow rapid access in the event of outbreaks with pandemic potential.
2. Benefit Sharing – During PHEICs and Pandemics
Article 12 currently sets a target of 20% VTD reserves for pandemic emergencies, with 10% guaranteed free of charge. As proposed in Element 1, extending these reserves to developing countries is essential to prevent PHEICs and pandemics.
Additionally, to ensure sufficient production during an emergency, manufacturers using PABS materials and sequences must grant manufacturing licenses to the WHO, which can then sublicense them to manufacturers in developing countries.
3. Benefit Sharing – Financial Contributions
Users of PABS Materials and revenue-generating sequences must make financial contributions.
These funds should be used to strengthen health systems in developing countries and develop geographically diverse VTD manufacturing and distribution capacities.
4. Legally Binding Contracts for Access to PABS Materials and Sequences
Only individuals/entities accepting benefit-sharing commitments through legally binding contracts should have access to PABS Materials and Sequences.
These contracts should specify that any access to the Materials and Sequences is subject to legal and specific conditions relating to the prevention, preparation and response to pandemics, with equitable sharing of the results obtained.
5. Scope of the PABS System
In accordance with Article 12, the scope of the PABS system must be limited to "pathogens with pandemic potential."
The use of materials and sequences must be for the sole purpose of preventing, preparing for, and responding to pandemics. Effective measures must prevent the reuse of resources for other purposes.
6. Traceability and Reporting Mechanism
For increased transparency and accountability, it is essential that all users of PABS Materials be identifiable and under a reporting obligation.
All transfers of PABS Materials, including those to WHO-designated laboratories, must be recorded.
Sequence databases must report to WHO and the Parties to the Agreement. No anonymous sharing.
All contracts, as well as financial and non-financial benefits received, must be made public.
7. Governance of the PABS System
WHO shall coordinate and administer the PABS system, under the supervision of the Conference of the Parties, including all the key elements mentioned above.
CHINA
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/China-en.pdf
Unofficial Translation
Comments and Suggestions of the China Regarding Elements to be Addressed in the Annex of the WHO Pandemic Agreement
According to the negotiation vision for the main text of the World Health Organization Pandemic Agreement, the dedicated Annex formulated under Article 12 will provide specific implementation details on the provisions for Pathogen Access and Benefit-Sharing (PABS), clarifying the obligations and responsibilities of PABS participants under the WHO multilateral system. To this end, the following comments and recommendations are proposed:
I. Guiding Principles Suggestions:
While safeguarding national biosafety and reaffirming States’ sovereign rights over their biological resources, every effort should be made to facilitate the sharing of pathogen-related information, maximise its public-health value, and promote equitable global access to vaccines, therapeutics and diagnostics arising from such sharing.
II. Participation in the PABS System Suggestions:
Define the scope of eligible participants and the modalities of their engagement, covering national governments, manufacturers of health products, research institutions, international organisations and philanthropic entities. For manufacturers participating in the PABS framework, specify qualification criteria, boundaries of liability, and both financial and technical benchmarks, and make these contingent on whether their home State is a Party to the Pandemic Agreement.
III. Definition of Pathogens with Pandemic Potential Suggestions:
A pathogen should qualify only if it is both highly transmissible and highly virulent, lacks effective countermeasures, and shows clear potential for cross-regional spread that could evolve into a global emergency. The definition should draw on WHO’s June 2024 publication “Pathogens Prioritisation – A Scientific Framework for Epidemic and Pandemic Research Preparedness” to establish a PABS pathogen list, while also taking into account the feasibility of manufacturing and access to related medical countermeasures so as to avoid an over-expansive list that would dilute focus.
IV. Governance of Genetic Sequence Data Suggestions:
Apply a tiered-management approach to GSD: sensitive data must be de-identified, access strictly controlled, and multiple technical safeguards deployed to prevent leakage or misuse, all in line with existing standards. Establish cross-platform sharing mechanisms, clarify rules on data use, keep basic data fully open, and allow high-risk information to be shared on a restricted basis after vetting. Build upon the three existing WHO databases (GISRS, FluNet and FluID) to extend platform coverage, strengthen the link between physical samples and digital data, and ensure full traceability.
V. Traceability of Pathogens Suggestions:
Institute a mechanism that tracks both the chain of custody of biological samples and the linkage to associated data, thereby creating a complete evidentiary trail. The Influenza Virus Traceability Mechanism (IVTM) can serve as a reference model.
VI. Data Openness:
Uphold the principles of controllable security, tiered and classified openness, and matching rights with responsibilities. High-risk data may be released only after review and only to accredited laboratories (endusers). Conditionally open data shall require certification or application; restricted data must be de-identified or shared solely with authorised entities. Non-sensitive baseline data should be fully open. End-users must commit to lawful use, provide timely feedback on results, and offer the providing party opportunities to participate in related research.
VII. Pathogen Transport and Biosafety Suggestions:
Guided by the principles of respect for sovereignty, risk stratification and global equity, establish a safe, equitable and efficient cross-border transport regime that facilitates pathogen and sample sharing while maintaining full traceability of biological material transfers. The regime should cover import/export approvals, packaging, transport and emergency-response requirements, and must align with domestic legislation and internationally recognised technical standards. Explore a global logistics coordination framework comprising multimodal corridors, strategic stockpile hubs, regional nodes, unified technical standards, standardised protocols, priority-clearance mechanisms, digital platforms and blockchain-based traceability. Ensure that all pathogen samples are handled in accordance with the WHO Laboratory Biosafety Manual and other relevant guidelines to mitigate laboratory biosafety risks.
VIII. Benefit Sharing Suggestions:
i) Ensure that benefits flow to the providing party. Create a feedback mechanism inspired by the Nagoya Protocol, grant priority access to resulting health products, and require attribution of contributions or the offer of collaboration opportunities.
ii) Develop quantified allocation criteria, allocation principles and oversight mechanisms for PABS benefit sharing, including sample handling fees, distribution mechanisms and workflows.
iii) Elaborate detailed rules for sharing pandemic-related health products, covering the scope of products, the timeframe for achieving target sharing ratios, applicable quality standards and risk-management measures.
iv) Clarify the recipients, necessity and calculation rules for annual contributions, assess possible adverse impacts and adopt appropriate safeguards.)
IX. Relationship with Other Instruments Suggestions:
Ensure coherence with other international instruments. The annex should include relevant provisions confirming that Article 12 creates no obligations for non-Parties to the Convention on Biological Diversity or the Nagoya Protocol, and imposes no additional obligations on institutions participating in the Pandemic Influenza Preparedness (PIP) Framework when accessing or utilising influenza or avian influenza viruses.
COLOMBIA
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Colombia.pdf
Draft as of 10 July 2025
Structural elements of Article 12 of the WHO Pandemic Agreement
Note - In general, the paragraphs of Article 12 of the WHO Pandemic Agreement are represented at the section level for ease of cross-reference and transparency.
Section A – Scope (Article 12.1)
The rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes.
Colombia – criteria:
Dispositions of the mechanism should not hinder information sharing, data openness, transparency and innovation and research efforts through the sharing of materials and sequence information of pathogens.
Section B – Elements to be developed in the PABS System (Article 12.2 and 12.5)
(1) Definitions (Article 12.2)
Pathogens with pandemic potential; (Article 12.2)
Colombia - Text Proposal:
Pathogens that represent, or could represent, a public health emergency of international concern, in the terms defined by the International Health Regulations (2005), and after applying the criteria contained in the Decision Instrument for the Assessment and Notification of Events that May Constitute a Public Health Emergency of International Concern.
Rationale:
The definition must be coherent with the International Health Regulations (2005) definition of “public health emergency of international concern” and its notification procedure, considering criteria such as risk significance, seriousness of the impact, among others.
PABS Materials and Sequence Information; (Article 12.2)
Colombia – Text Proposal:
- PABS Materials: Pathogen with pandemic potential and or its physical parts and components, including DNA, RNA, proteins, among others.
- PABS Sequence Information: Information derived from the analysis of PABS materials.
Comment:
It is essential that both elements (materials and sequence information) are separately defined.
Participating manufacturer (Article 12.6(a))
Other technical terms
(2) Modalities, terms and conditions on access and benefit sharing that provide legal certainty (Article 12.5(b))
(3) Legal nature (Article 12.2)
(4) Coordination and operation of the PABS System, in collaboration with relevant international organizations and relevant stakeholders (Article 12.2)
(5) Administration and coordination of the PABS System by WHO (Article 12.2)
Colombia – criteria:
The management and administration of the PABS system must involve focal point of country Parties and must have oversight and accountability mechanisms to ensure the adequate use of resources.
(6) Sharing of material and sequence information and of benefits, both monetary and nonmonetary, including annual monetary contributions and vaccines, therapeutics and diagnostics (Article 12.5(a))
Colombia – criteria:
Dispositions of the mechanism should not hinder information sharing, data openness, transparency and innovation and research efforts through the sharing of materials and sequence information of pathogens.
(7) Facilitation and acceleration of research and innovation (Article 12.5(c))
Colombia – criteria:
PABS mechanism should not hinder Parties’ autonomy to share PABS materials and sequence information in efforts associated to innovation and research.
Emphasis should be made in developing countries needs and possible mechanisms of support such as technologies and knowledge transfer agreements.
(8) Complementarity with the PIP Framework and other relevant ABS instruments (Article 12.5(d)(i))
Colombia – criteria:
PABS mechanism should not exclude other ABS instruments that include PABS materials and sequence information.
(9) Review and alignment of national and/or regional ABS measures (Article 12.5(d)(ii))
(10)Consistency with applicable law related to risk assessment, biosafety, biosecurity and export control of pathogens, and data protections (Article 12.5(e))
(11)Facilitation of manufacture and export of vaccines, therapeutics and diagnostics (Article 12.5(f))
Colombia – criteria:
Mechanisms of knowledge and technology transfer should be detailed, including import and export arrangements to facilitate access to relevant technologies during a pandemic.
Section C – Traceability and open access to data (Article 12.3)
Colombia – Text proposal:
Any traceability measure cannot go against the sharing of information, including public information or sequence information, and should maintain the active communication and collaboration and the sharing of information with public health significance
Rationale:
Measures taken cannot go against free access to information and data bases and must not favor the creation of private data bases. Free sharing of information must be ensured, respecting national legislation and each State’s autonomy.
Section D – Consistency with Nagoya Protocol (Article 12.4)
Colombia – criteria:
PABS must be compatible with article 15, paragraph 1 of CBD, and with article 3 of Nagoya.
Section E – Provisions in the event of pandemic emergency (Article 12.6)
(1) Rapid access to real-time production of vaccines, therapeutics and diagnostics (Article 12.6(a))
(2) Distribution of vaccines, therapeutics and diagnostics based on public health risks and needs, with particular attention to the need of developing countries (Article 12.5(b))
Section F – Provisions in the event of a public health emergency of international concern (Article 12.7)
Colombia – criteria:
Section must remain consistent with article 12 of the International Health Regulations (2005). The risk assessment of the event at the international level and the effectiveness of control measures should be considered. Measures to prevent and avoid national spread should be included.
Section G – Additional benefit-sharing provisions (Article 12.8)
Colombia – criteria:
This section should address considerations for the effective implementation of mutually agreed forms of technology transfer, including the transfer of relevant knowledge, skills, and technical expertise, in accordance with the provisions of Article 12.8(e).
Section H – Other elements for effective operationalization of the PABS System (Article 12.9)
EUROPEAN UNION
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/EU.pdf
EU input, 25 July 2025
OUTLINE OF ELEMENTS TO BE ADDRESSED BY THE ANNEX TO THE WHO PANDEMIC AGREEMENT DESCRIBED IN ARTICLE 12 OF THE WHO PANDEMIC AGREEMENT (THE “PABS ANNEX”)
Introductory remarks
In response to the invitation by the Bureau of the IGWG to submit initial text proposals, in particular the elements to be addressed by the Annex of the WHO Pandemic Agreement, the EU and its Member States submit the below input for the purpose of supporting the work of the Bureau to develop a draft outline of elements to be addressed by the PABS Annex. The elements set out below derive from Article 12 of the Pandemic Agreement and are essential to develop a well-functioning PABS System.
The elements can usefully be grouped into five main themes or areas:
Use of terms
Benefit sharing
Access
Governance issues and
General and final provisions.
To facilitate and advance work on the Annex in the best possible way, the EU and its MS consider that an enhanced common understanding of the overall purpose of the PABS, as well as of the eventual content of these five key areas will be beneficial. In this respect, the EU and its Member States look forward to constructive substantive discussions to this end at the second meeting of the IGWG in September.
We underline that the below input is initial and entirely without prejudice to further input and proposals, including proposals for legal text, that the EU and its Member States may decide to submit subsequently during the process to develop the PABS Annex.
1. USE OF TERMS
“Pathogens with pandemic potential”
“PABS Materials”
“PABS Sequence Information”
“WHO PABS System” (understanding of what “System” means and what is its legal nature)
Some of the parameters listed under Benefit Sharing and Access in particular may become defined “terms”
2. BENEFIT SHARING
Benefit Sharing parameters:
Contracts with participating manufacturers (which are both legally-binding and voluntarily concluded) as the instrument for benefit sharing
Specification of parameters (model clauses can be set out in an Appendix to the Annex if considered useful) for the making available of set aside quantities of VTDs to WHO, including:
scope of VTDs [Vaccines, Therapeutics and Diagnostics]
set aside quantities
triggering event (declaration of pandemic emergency)
rapid access
real time production
participating manufacturer
flexibility based on the nature and capacity/ size of each participating manufacturer ✓ donation ✓ reservation at affordable prices, etc.
Definition and parameters for quantification of annual monetary contribution o The distribution and use of annual monetary contributions
Options for benefit sharing in the event of a PHEIC
Definition of additional benefit sharing options (including in times of neither PHEIC nor PE):
capacity-building and technical assistance; o research and development cooperation;
facilitating rapid access to available vaccines, therapeutics and diagnostics with a view to responding to public health risks and events in the context of Article 13.3 of the International Health Regulations (2005);
granting of non-exclusive licences to manufacturers in developing countries, for the effective production and delivery of vaccines, therapeutics and diagnostics;
other forms of transfer of technology as mutually agreed, including transfer of relevant knowledge, skills and technical expertise.
Distribution of VTDs:
Definition of parameters and criteria for the equitable distribution of vaccines, therapeutics, and diagnostics based on public health risk and need;
Facilitation of the manufacture and export of vaccines, therapeutics and diagnostics for pathogens covered by the PABS Annex
3. ACCESS
Provisions to ensure “rapid and timely sharing” of PABS Materials (obligations for Parties)
Modalities and requirements for sharing (including laboratory requirements for handling materials, mechanisms for rapid shipment of materials, etc)
- Provisions to strengthen, facilitate and accelerate research and innovation
- Provisions to ensure “rapid and timely sharing” of PABS Sequence Information (obligations for Parties)
Modalities and requirements for sharing (including mechanisms to enhance analysis of SI, etc)
- Provisions to strengthen, facilitate and accelerate research and innovation
4. GOVERNANCE ISSUES
Terms for the administration and coordination of the PABS System by the World Health Organization
Collaboration with relevant international organizations and relevant stakeholders
Modalities for collaboration
Possible dedicated consultative body
Role of the Conference of the Parties and possible future periodic reviews of the PABS System.
5. GENERAL AND FINAL PROVISIONS
Relationship / consistency of PABS System with other international instruments or with applicable domestic law
Requirements to ensure that the PABS Annex shall be consistent with, and not run counter to, the objectives of the Convention on Biological Diversity and the Nagoya Protocol
Requirements to ensure that national and/or regional ABS measures that are contrary to or inconsistent with or duplicative of the PABS System will not be applied
Consistency with applicable international law and with applicable national and/or domestic law, regulations and standards related to risk assessment, biosafety, biosecurity and export control of pathogens, and data protection
Legal relation with the PIP Framework
Legal relation with other relevant international access and benefit sharing instruments (in particular the multilateral mechanism for DSI on genetic resources)
Provisions aimed at facilitating and accelerating research and innovation
Open access to data
Traceability (including purpose, scope, technical feasibility and cost)
Entry into operation (“All elements of the PABS System shall come into operation simultaneously in accordance with the terms of the PABS Instrument”) o Criteria for the entry into operation.
GROUP FOR EQUITY
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Group_for_Equity.pdf
SUBMISSION BY THE GROUP FOR EQUITY
OUTLINE & KEY ELEMENTS FOR ANNEX ON WHO PATHOGEN ACCESS AND BENEFIT SHARING SYSTEM (PABS SYSTEM)
This submission contains some elements to be addressed by the Annex, and is without prejudice to any further elements and textual proposals and suggestions put forward jointly or individually as the discussion progresses.
A. OBJECTIVE
This Annex is developed pursuant to Article 12 of the Pandemic Agreement and establishes the provisions governing the PABS System, in order to operationalize a fair, transparent, and accountable multilateral access and benefit-sharing system for PABS materials and sequence information.
B. SCOPE
1. The Annex applies to the rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information.
2. For the purpose of the Annex “pathogen with pandemic potential” means [placeholder]
3. The PABS System shall operate complementarily to the PIP Framework.
4. All elements of the PABS System shall come into operation simultaneously.
C. DEFINITIONS FOR THE PURPOSE OF THE ANNEX
(the list below is non-exhaustive and may be expanded)
1. PABS Materials: (i) isolated wild-type pathogens assessed to have pandemic potential, and parts thereof; (ii) any modified forms of such pathogens; and (iii) any other materials derived from, generated or prepared using the materials described in (i) and (ii).
2. PABS Sequence Information: any data or information generated from PABS Materials through the application of sequencing technologies
3. Authorized national laboratories: laboratories authorized and designated by a Party to provide PABS Materials and/or PABS Sequence Information to the PABS System and recognized as part of the WHO Coordinated Laboratory Network.
4. Clinical Specimens: materials taken from humans including specimens collected from the respiratory tract (for example, swabs and aspirated fluid), and also blood, serum, plasma, faeces, and tissues.
5. Originating laboratory: a national authorised laboratory that sent the initial clinical specimens and/or the isolated pathogen with pandemic potential
6. Participating Manufacturer: public and/or private entities that develop and/or manufacture vaccines, therapeutics and diagnostics for pathogens with pandemic potential including academic institutions, government owned or government subsidized entities, nonprofit organizations or commercial entities.
7. WHO Coordinated Laboratory Network an international network of authorized national laboratories coordinated by WHO and governed by the PABS System under the supervision of COP. To be a part of the WHO Coordinated Laboratory Network, an authorized national laboratory must meet the criteria for designation and agree to comply with terms and conditions as set out in the Standard Contract 1, the applicable Terms of Reference, and any other requirements of the PABS system.
8. [placeholder: affordable prices]
D. FRAMEWORK OF THE PABS SYSTEM
(Colombia requires further internal consultation on section D, sub-sections 1, 2, 3, and 5.)
1. WHO Coordinated Laboratory Network (WCLN)
a. WCLN coordinated by WHO under the oversight of COP, shall be established. The network will link authorised national laboratories that meet the criteria for participating in the WCLN and agree to accept the Terms of Reference (ToR), the legally binding terms and conditions applicable to the sharing/utilization of PABS Materials and PABS Sequence Information, as well as other requirements of the PABS system.
b. WHO shall be responsible for facilitating the funds required to cover the costs related to shipment of PABS Materials to and within the WHO Coordinated Laboratory Network, by developing country Parties.
2. Standard Legally Binding Contracts Applicable to the Sharing of PABS Materials and PABS Sequence Information
a. All transfers of PABS Material by an authorised national laboratory to a laboratory that is part of WCLN and further transfers within WCLN are subject to Standard Contract 1.
b. By providing PABS Materials to laboratories designated as part of the WHO Coordinated Laboratory Network, the provider Party provides their consent for the onward transfer to and use of PABS Materials by Recipient Entities outside the WCLN that have signed Standard Contract 2 with WHO. Recipients agree not to transfer or share PABS materials to entities outside WCLN that have not signed Standard Contract 2.
c. Both Standard Contract 1 and 2 shall contain terms and conditions applicable to the storage, sharing, and use of sequence Information generated by recipients from PABS Materials
3. PABS Sequence Information & Traceability Measures
3.1. WHO Coordinated Database Network (WCDN)
a. Sharing of any PABS sequence information shall be through a WHO PABS Sequence Database that is transparent and accountable to Parties and implements requirements set out in subparagraph (d) and COP decisions. WHO may host such a database or may, following a COP decision, organise an existing database to host the WHO PABS Sequence Database.
b. Other databases may access and share PABS sequence information, subject to the database entering into a legally binding contract with WHO, agreeing to comply with terms and conditions set out in sub-paragraph (d) (hereinafter referred to as “Participating Databases”).
c. WHO PABS sequence database, along with participating databases constitute WCDN. d. WHO PABS Sequence Database and other Participating Databases hosting PABS Sequence Information to comply with terms and conditions including:
(i) require registration and acceptance of Data Access Agreement (Standard Contract 3) from all users of database intending to access PABS Sequence Information;
(ii) provide access to PABS Sequence Information to all registered users, whose identity is verified, without any fee charged and without any form of discrimination.
(iii) attach persistent unique labels to sequence files or datasets in a manner identifying originating country of the PABS materials from which sequence information is generated
(iv) make available facilities for registered users to link their scientific or other publications, provide information of R&D progress and R&D outcomes that use/refer to PABS Sequence Information;
(v) provide information related to usage of databases, including a list of registered users as well as other particulars, to WHO periodically and/or upon request.
(vi) agree to comply with all requirements of PABS system and act in accordance with WHO/COP decisions in this regard.
(vii) In the event, the participating database is generating revenue from the sharing/utilization of PABS Sequence Information, the participating database agrees to contribute towards monetary benefit-sharing;
3.2 Standard Legally Binding Contract Applicable to the Sharing of PABS Sequence Information
a. Parties to take measures to ensure that PABS sequence information is shared through the WHO PABS Sequence Database and consistently with its requirements.
b. Laboratories within WCLN to share all PABS Sequence Information through the WHO PABS Sequence Database.
c. Submission of PABS Sequence Information to be based on the format as decided by the COP, and such format shall include requiring the submitter to provide information on the country of the originating laboratory
4. Fair and Equitable Benefit-Sharing from Recipients of PABS Materials and Sequence Information (to be reflected in Standard Contracts 2 and 3)
4.1 Annual Monetary Contributions (applicable to all recipients accessing PABS Materials and/or PABS Sequence Information)
When revenue is generated from the sharing/utilization of PABS Materials and/or PABS Sequence Information, to annually contribute x% of total annual revenue for each product or service developed and commercialised using the PABS System. Annual revenue includes all financial benefits such as income from sales and royalties.
4.2 Non-monetary Benefit Sharing
a. Participating manufacturer:
(i) in the event of a pandemic emergency, to make available make available to the WHO rapid access targeting 20% of their real time production of safe, quality and effective vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency, provided that a minimum threshold of 10% of their real time production is made available to the WHO as a donation, and the remaining percentage, with flexibility based on the nature and capacity of each participating manufacturer, is reserved at affordable prices to the WHO, in accordance with paragraph 6 of Article 12
(ii) to provide to WHO on request, at not-for-profit prices and on a priority basis, the vaccines, therapeutics and diagnostics, for stockpiling and/or to supply affected developing countries, to prevent, prepare for and respond to a PHEIC. The set-asides to be determined in IGWG
(iii) in the event of a PHEIC and/or a pandemic emergency, to grant WHO non-exclusive licenses that can be sublicensed to manufacturers in developing countries, especially to those that provided PABS Materials and PABS Sequence Information, for the development and/or production of pandemic-related products needed in developing countries, to expand supply rapidly, ensuring prompt equitable access in developing countries. Such a license to include provision of the full regulatory dossier, technical know-how, and any necessary materials (e.g. cell-lines etc.).
b. Recipients accessing PABS Materials and/or PABS Sequence Information for strictly for Non-Commercial uses (not intended to generate revenue)
To make available all outcomes from the non-commercial use of PABS Materials and PABS Sequence Information freely available and accessible in the public domain, and the same shall be reported through the WHO PABS Sequence Database such that it shall be linked to the sequence information accessed. In the event revenue is generated, monetary benefit sharing will be required.
c. Other Recipients
[placeholder to add further non-monetary benefit sharing as discussion progresses]
5. Transparency, Traceability & Reporting Mechanism
5.1 Access to PABS Materials and Sequence Information shall be subject to the prior informed consent of the Party providing such resources, and mutually agreed terms between providers and users of such resources.
5.2 WHO to establish a PABS tracking Mechanism to record all transfers of PABS Materials
5.3 WHO to make publicly available:
a. the list of registered recipients of PABS Sequence Information;
b. the list of WCLN labs
c. monetary contributions received from each recipient and their utilization;
d. standard contracts 2 & 3 concluded with each recipient.
6. Governance of the PABS System
6.1. PABS system to be coordinated and administered by the WHO, under authority of the COP. WHO to take all measures necessary to ensure a fair, transparent, equitable, effective, efficient and accountable implementation of the PABS System.
6.2. An independent, free of conflict of interests, oversight “PABS Advisory Group” to be established to monitor and to provide guidance to WHO on the functioning of the PABS system in accordance with the terms of reference for the Advisory Group. The PABS Advisory Group to present an annual report to the COP on its evaluation of the implementation of the PABS system, with its recommendations for COP’s approval.
6.3. The Director-General, in consultation with Member States, to ensure that the PABS Advisory Group is based on fair and equitable representation of the WHO regions, taking into account the importance of representation from developing countries. The PABS Advisory Group will comprise xx members drawn from each WHO Region, with a skill mix of internationally recognized policy makers, public health experts and technical experts.
6.4. ?
6.5. In the event of any alleged breaches of the terms of reference or the Standard Contract by a WCLN laboratory, the Director-General will review the circumstances and discuss with the PABS Advisory Group any appropriate action in response to those breaches. Where there has been a serious breach, the Director-General may consider suspending or revoking the WHO designation of the relevant laboratory.
6.6 In the event of any alleged breaches of the terms of reference or the Standard Contract by WHO PABS Sequence Database or participating databases, the Director-General will review the circumstances and discuss with the PABS Advisory Group any appropriate action in response to those breaches. Where there has been a serious breach, the Director-General may consider suspending or revoking the WHO designation of the relevant database.
6.7. In the event of any alleged breaches of the Standard Contract by receiving entities (outside the WCLN laboratory, WHO PABS Sequence Database, and participating database), the Director-General will review the circumstances and discuss with the PABS Advisory Group any appropriate action in response to those breaches. Where there has been a serious breach, the Director-General may consider suspending or revoking the access of such receiving entities.
6.8. Responsibilities of Parties:
a. to take all steps to facilitate the rapid and timely manufacture and export of vaccines, therapeutics and diagnostics, and to operationalise other benefit-sharing obligations that recipients have committed to provide to prevent, prepare for and to respond to a PHEIC and/or a pandemic emergency for pathogens covered by the PABS system.
b. to ensure compliance of non-state actors with all components of the PABS System
E. STANDARD LEGALLY BINDING CONTRACTS
STANDARD CONTRACT 1: TERMS AND CONDITIONS APPLICABLE TO NATIONAL AUTHORISED LABORATORIES AND WCLN LABORATORIES
Legally binding contract between the Provider (national authorised laboratory sending PABS Materials and Sequence Information) and Recipient (WCLN laboratory receiving PABS Materials and Sequence Information), to be concluded when becoming a member of WCLN. Terms and conditions to be included in the standard contract include:
1. The recipient agrees to comply with its ToR and to use the PABS Material and clinical specimen solely for public health purposes as set out in the Terms of Reference, for the prevention, preparedness and response to a pandemic, and not-for-profit.
2. All transfers of PABS Materials to be recorded in the PABS Materials Tracking Mechanism.
3. Onward transfer and use of the PABS Materials to all WCLN laboratories to be on the same terms and conditions applicable to WCLN; and to entities outside the WCLN subject to the Entity concluding Standard Contract 2 with the WHO.
4. Recipient to actively seek participation of scientists from originating laboratories, especially those from developing countries, in scientific or R&D projects associated with research on PABS Material and Sequence Information from their countries, as well as actively engage them in preparation of manuscripts for presentation, publication or any other outcomes;
5. Recipient to appropriately acknowledge in presentations and publications the contributions of collaborators, including originating laboratories/countries providing the PABS Material and Sequence Information.
6. Intellectual property shall not be sought or asserted over any PABS Materials and Sequence Information, or parts thereof, in any form, including any modified form or for any use.
7. The recipient to regularly and/or on request, share all analyses and outcomes from the sharing/utilization of PABS Materials and PABS Sequence Information with the Provider.
8. Sharing of PABS sequence information to be through the WHO PABS Sequence Database and agree to comply with the terms and conditions as applicable to the users of PABS Sequence Information.
9. Agrees to comply with COP decisions with respect to the PABS system
10. Criteria for an authorized national laboratory to ensure biosafety and biosecurity.
STANDARD CONTRACT 2:
TERMS AND CONDITIONS APPLICABLE BETWEEN WHO AND ENTITIES OUTSIDE OF WCLN
Legally binding contract between: WHO and the Recipient Entity (outside of the WCLN laboratory receiving PABS Materials and PABS Sequence Information). The recipient entity can access PABS Materials and PABS Sequence Information from WCLN, subject to the conclusion of a legally binding contract with WHO. Terms and conditions of the standard contract include:
1. using the PABS Materials and PABS Sequence Information solely and strictly for research and development in connection with pathogens with pandemic potential within the scope of the PABS system for the prevention, preparedness and response to a pandemic.
2. to keep WHO informed of all uses of PABS Materials and PABS Sequence Information, as well as any changes to their intended use. Any use of PABS Materials and Sequence Information outside the scope of the PABS System must obtain prior informed consent from the originating country.
3. transferring the PABS Materials only if the prospective recipient has concluded the required contract with WHO. Any such transfer shall be reported to the WHO through the PABS Tracking Mechanism.
4. sequence information generated by the Recipient from the PABS Materials, sharing/transfer through WHO PABS Sequence Database and recipient to comply with terms of data access agreement (standard contract 3)
5. not to seek or assert intellectual property over any PABS Materials and PABS Sequence Information, or parts thereof, in any form, including any modified form or for any use.
6. to comply with monetary and non-monetary benefit-sharing obligations
7. Agrees to comply with COP decisions with respect to the PABS system
STANDARD CONTRACT 3: DATA ACCESS AGREEMENT (may potentially be in a form of a clickwrap agreement)
TERMS AND CONDITIONS APPLICABLE TO ANY PERSON/ENTITY ACCESSING PABS SEQUENCE INFORMATION FROM WHO PABS SEQUENCE DATABASE/PARTICIPATING DATABASES
Legally binding contract for parties - the Database, WHO and person/entity seeking access to PABS Sequence Information. Access to PABS Sequence Information is subject to registration and acceptance of data access agreement with terms that include:
1. use solely and strictly for research and development in connection with pathogens with pandemic potential within the scope of the PABS system for the prevention, preparedness and response to a pandemic;
2. not be used in any activity that may lead to the development, or production of biological agents, toxins, weapons, equipment, or means of delivery specified in Biological Weapons Convention;
3. transfer or share sequence information only if the prospective recipient is also a registered user either of the WHO PABS Sequence Database or a Participating Database;
4. acknowledge the contributions of the persons and institutions who submitted the sequence information as well as the originating laboratories that first collected the clinical specimen and isolated the pathogen with pandemic potential;
5. actively seek the participation of scientists from originating laboratories/countries of PABS Materials and clinical specimens from which the PABS Sequence Information was generated, especially those from developing countries, in scientific or R&D projects associated with the PABS Sequence Information, and to actively engage them in the development and implementation of the projects;
6. not seek or assert intellectual property over PABS Sequence Information or parts thereof, in any form, including any modified forms or for any use;
7. Agrees to comply with the monetary and non-monetary benefit-sharing obligations.
8. Agrees to comply with COP decisions with respect to the PABS system.
INDONESIA
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Indonesia.pdf
PERMANENT MISSION OF THE REPUBLIC OF INDONESIA TO THE UNITED NATIONS WORLD TRADE ORGANIZATION (WTO) AND OTHER INTERNATIONAL ORGANIZATIONS IN GENEVA
No. 126/POL-II/VIII/2025
The Permanent Mission of the Republic of Indonesia to the United Nations, World Trade Organization, and other International Organizations in Geneva presents its compliments to the World Health Organization and has the honor to transmit herewith the initial text proposals of the Republic of Indonesia for the outline and elements of the Annex on Pathogen Access and Benefit Sharing of the WHO Pandemic Agreement. The Permanent Mission of the Republic of Indonesia to the United Nations, World Trade Organization and other International Organizations in Geneva avails itself of this opportunity to renew to the World Health Organization the assurances of its highest consideration.
Geneva, 26 August 2025
World Health Organization Secretariat, Governing Bodies Geneva
INITIAL TEXT PROPOSALS BY THE REPUBLIC OF INDONESIA TO THE INTERGOVERNMENTAL WORKING GROUP
This submission is without prejudice to any subsequent proposals and/or modifications as the discussion progresses.
ON THE DRAFT OUTLINE AND ELEMENTS FOR ANNEX ON PATHOGEN ACCESS AND BENEFIT-SHARING
1. Principles
1.1 Recognition of the sovereign right of States over their own biological resources.
1.2 Rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) on an equal footing with the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information.
1.3 Rapid, timely, fair and equitable access to safe, quality, and effective vaccines, therapeutics, and diagnostics.
1.4 Safe, transparent, and accountable access and benefit-sharing for PABS Materials and Sequence Information.
1.5 Access to genetic resources shall be subject to the prior informed consent of the Parties providing such resources, and, where granted, shall be on mutually agreed terms.
2. Objective
This Annex is developed pursuant to Article 12 of the WHO Pandemic Agreement and establishes the provisions governing the PABS System, in order to operationalize a multilateral system for safe, transparent, and accountable access and benefit-sharing for PABS Materials and Sequence Information.
3. Scope
This Annex applies to the rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (PABS Materials and Sequence Information) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes.
4. Definitions / Use of terms for the purpose of this Annex
4.1 “Pathogens with pandemic potential” refers to any pathogen that is:
novel (not yet characterized) or known (including a variant of a known pathogen),
potentially highly transmissible within human populations,
can cause a public health emergency of international concern (PHEIC) and/or a pandemic emergency, as defined in the International Health Regulations (IHR). A list of pathogens with pandemic potential may be developed and periodically updated, based on available scientific evidence, surveillance data, and global health joint risk assessments, as well as the use of the WHO R&D Blueprint Priority Pathogens.
4.2 “PABS Materials” refers to isolated wild-type pathogens assessed to have pandemic potential, any modified forms of such pathogens, and any other materials derived from, generated or prepared using the PABS Materials.
4.3 “PABS Sequence Information” refers to any data or information generated from PABS Materials through the application of sequencing technologies.
4.4 “Authorized national laboratories” refers laboratories authorized and designated by a Party to provide PABS Materials and/or PABS Sequence Information to the PABS System. These laboratories are recognized as part of the WHO-Coordinated Laboratory Network (WCLN).
4.5 “WHO-Coordinated Laboratory Network” (WCLN) refers to an international network of authorized national laboratories, coordinated by WHO and governed by the PABS System under the guidance of the Conference of the Parties (COP). Authorized national laboratories may become part of the WCLN by meeting its criteria for designation and accepting its Terms of Reference as well as terms and conditions set out in Standard Material Transfer Agreement (SMTA) 1.
4.6 “Clinical specimens” refers to materials taken from humans, including specimens collected from the respiratory tract (for example, swabs and aspirated fluid), and also blood, serum, plasma, faeces, and tissues, as well as those collected from wastewater treatment.
4.7 “Participating manufacturers” means public and/or private entities that develop and/or manufacture vaccines, therapeutics, and diagnostics for pathogens with pandemic potential, including academic institutions, government-owned or government subsidized entities, non-profit organizations or commercial entities, and has entered into a legally binding contract/agreement with WHO for accessing PABS Materials and Sequence Information.
4.8 “Affordable prices” shall be determined, based on the verified cost of production plus a reasonable margin not exceeding ...%, in a manner that is consistent with, and does not run contrary to, the objective of ensuring equitable access to safe, quality, and effective vaccines, therapeutics, and diagnostics for the pathogens causing the pandemic emergency.
4.9 “Real-time production” refers to [placeholder]
5. General Terms and Conditions of Access
5.1 Access to genetic resources shall be subject to the prior informed consent of the Parties providing such resources, and, where granted, shall be on mutually agreed terms
5.2 Recipient entities may access PABS Materials and Sequence Information only upon the conclusion of a Standard Material Transfer Agreement (SMTA) and a Data Access Agreement (DAA).
5.4 Rapid and timely sharing of PABS Materials and Sequence Information shall take into account the capacities and circumstances of developing and least developed countries.
6. Framework of the PABS System
6.1 WHO-Coordinated Laboratory Network (WCLN)
a. WCLN shall be established under WHO coordination and CoP oversight.
b. WCLN shall comprise authorized national laboratories meeting designation criteria and accepting the Terms of Reference (ToR) and SMTA 1.
c. WHO shall facilitate the funding for shipment of PABS Materials to and within the WCLN by developing countries.
d. Transfers to a laboratory that is part of WCLN, and further transfers within WCLN, shall be subject to the conclusion of SMTA 1.
e. Transfers outside the WCLN shall be subject to the conclusion of SMTA 2.
f. WHO shall make publicly available list of WCLN laboratories and SMTAs concluded.
g. The WCLN works shall be included the Annual Report of the PABS Advisory Group
6.2 WHO PABS Sequence Database
a. PABS Sequence Information shall be shared through a WHO PABS Sequence Database, either hosted by WHO or designated by the CoP.
b. Access to WHO PABS Sequence Database requires user registration, identity verification, and acceptance of Data Access Agreement (DAA), including benefit sharing obligations.
c. Verified registered users shall have non-discriminatory access upon accepting the DAA. Such access will not be anonymous.
d. WHO shall make available list of registered and verified recipients of PABS Sequence Information, as well as Data Access Agreement concluded.
e. The works of the WHO PABS Sequence Database shall form an integral part of the Annual Report of the PABS Advisory Group.
6.3 Traceability Measures
a. PABS Materials Tracking Mechanism shall record and track the transfer and movement of PABS Materials into, within and out of the WCLN, as well as their storage and usage.
b. Access to WHO PABS Sequence Database requires user registration, identity verification, and acceptance of DAA. PABS Sequence Information shall carry persistent identifiers, linking it to the providers of PABS Materials from which the Sequence Information was generated. Removal of such identifiers is prohibited.
6.4 Standard Material Transfer Agreement (SMTA) 1 – a legally binding contract between authorized national laboratories (providers of PABS Materials and Sequence Information) and the WCLN (recipients of PABS Materials and Sequence Information). SMTA 1 shall contain the following provisions:
a. PABS Materials and Sequence Information are provided strictly for public health and not-for-profit purposes. Uses beyond are prohibited.
b. All transfers of PABS Materials shall be recorded in the PABS Materials Tracking Mechanism.
c. Providers consent to onward transfer within WCLN under SMTA 1 and outside WCLN upon the conclusion of SMTA 2.
d. Recipients shall appropriately acknowledge originating laboratories or countries.
e. Recipients shall not seek intellectual property rights over PABS Materials and Sequence Information, or parts thereof, in any form, including any modified form.
f. PABS Sequence Information shall be shared through WHO PABS Sequence Database, in compliance with Data Access Agreement (DAA).
g. Criteria for an authorized national laboratories to ensure biosafety and biosecurity.
h. [placeholder] Issues related to liability and indemnity.
i. [placeholder] Dispute resolution.
6.5 Standard Material Transfer Agreement (SMTA) 2 – a legally binding contract between WHO and entities outside the WCLN SMTA 2 shall contain the following provisions:
a. PABS Materials and Sequence Information shall be used strictly for research and development, including the development of vaccines, therapeutics, and diagnostics for the pathogens with pandemic potential.
b. Any use outside the scope of the PABS System must obtain prior informed consent of the originating countries, and notification to WHO.
c. Any transfer of PABS Materials to entities outside WCLN is subject to the conclusion of SMTA 2, and shall be recorded in PABS Material Tracking Mechanism.
d. PABS Sequence Information shall be shared through WHO PABS Sequence Database, in compliance with Data Access Agreement (DAA).
e. Recipients shall comply with benefit sharing obligations.
f. Recipients shall not seek intellectual property rights over PABS Materials and Sequence Information, or parts thereof, in any form, including any modified form.
g. [placeholder] Dispute resolution.
6.6 Data Access Agreement (DAA) – a legally binding agreement for entities seeking access to PABS Sequence Database DAA shall contain the following provisions:
a. PABS Sequence Information shall be used strictly for research and development, including the development of vaccines, therapeutics, and diagnostics for the pathogens with pandemic potential.
b. Recipients shall appropriately acknowledge persons and institutions contributing PABS Sequence Information.
c. Onward transfer is permitted only to registered users of WHO PABS Sequence Database.
d. Recipients shall comply with benefit sharing obligations.
e. Recipients shall not seek to intellectual property rights over PABS Materials and Sequence Information, or parts thereof, in any form, including any modified form.
f. [placeholder] Dispute resolution.
6.7 Biosafety and biosecurity
a. Stringent biosafety and biosecurity measures shall apply to the collection, transportation, storage and experiment to research and development activities, repository and biobank, using laboratories with biosafety levels ....
b. [placeholder] Provisions on biosafety and biosecurity may also address:
Infrastructure and human resource capacity.
Compliance with applicable national and international norms and standards.
International collaboration, including capacity-building.
7. Benefit Sharing
7.1 Annual monetary contributions
a. All recipients accessing PABS Materials and Sequence Information shall make annual monetary contributions, amounting to …% of revenue from products or services developed and commercialized using the PABS System.
b. WHO shall manage annual monetary contributions collected in a transparent and equitable manner to improve pandemic prevention, preparedness, and response, including manufacturing capacity in developing countries.
c. The use of annual monetary contributions collected shall be decided by WHO under the CoP guidance.
d. For greater transparency and accountability, WHO shall make publicly available details of annual monetary contributions collected and their allocation.
7.2 Non-monetary benefit-sharing
a. Participating manufacturers:
In the event of a pandemic emergency: To make available vaccines, therapeutics, and diagnostics in accordance with paragraph 6 of Article 12.
Low- and middle-income countries providing PABS Materials and/or PABS Sequence Information shall be entitled to host multi-country clinical trials conducted by participating manufacturers. Such entitlement shall be subject to applicable national laws and regulations, and aimed at enhancing regulatory and scientific capacity and facilitating timely access to vaccines, therapeutics, and diagnostics.
In the event of a Public Health Emergency of International Concern: To make available ...% of real-time production of relevant vaccines, therapeutics, and diagnostics to WHO.
In the event of public health risks and events: To share benefits in accordance with paragraph 8 of Article 12, and [placeholder].
b. Recipients accessing PABS Materials and/or PABS Sequence Information for non-commercial purpose shall make available all resulting outcomes freely available and accessible.
c. Other non-monetary benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information shall include:
Collaboration in Research and Development, particularly with institutions from developing countries.
Participation of pharmaceutical companies in developing countries in product development.
Training and capacity-building, especially with research institutions from developing countries.
Technology transfer, including timely access to vaccine seed strains and other essential material for broader production of vaccines, therapeutics, and diagnostics.
Licences to manufacturers in countries, or neighboring countries, affected the most by the PHEIC and pandemic emergency.
d. Allocation and distribution of vaccines, therapeutics, and diagnostics:
WHO, under the guidance of the CoP, shall establish transparent criteria for allocating vaccines, therapeutics, and diagnostics.
Allocation shall be based on public health risk and need, with priority to developing countries, considering epidemiological trends, socioeconomic impacts, and their domestic production capacity.
WHO shall make publicly available details of vaccines, therapeutics, and diagnostics made available by participating manufacturers, along with information on their allocation and distribution.
8. Governance of the PABS System
8.1 The PABS System shall be administered and coordinated by the WHO, under the authority and oversight of the CoP. WHO Secretariat shall serve as the PABS System Secretariat.
8.2 An independent, free of conflict-of-interests “PABS Advisory Group” shall monitor and guide effective implementation of the PABS System.
8.3 The Director-General, in consultation with Member States, shall ensure fair and equitable representation of the WHO regions and a balance of developed and developing countries in PABS Advisory Group, with members drawn from policy, public health, and technical expertise.
8.4 Members of PABS Advisory Group shall declare any conflict of interests, and WHO shall make such declaration publicly available.
8.5 The PABS Advisory Group may review and recommend adjustment to SMTAs, DAAs, and amount of annual monetary contributions, for the consideration of the CoP.
8.6 The PABS Advisory Group shall submit an annual report to the Director-General on the operation of the PABS System, including compliance with benefit sharing obligations.
8.7 Member States may report allegations of breaches of SMTA or DAA by recipient entities. The Director-General shall review the circumstances with the PABS Advisory Group, and, in serious cases, may consider suspending or revoking access of such recipient entities.
8.8 Parties shall ensure compliance with all components of the PABS System, including benefit-sharing obligations.
JAPAN
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Japan.pdf
Submission by Japan
August 8, 2025
Key structural issues to be addressed for the PABS system
Based on the discussion at the first and organizational meeting of the IGWG in July 2025 and in pursuance of paragraph 2 of A/IGWG/1/3 Rev.1 (“Timeline and deliverables of the open-ended IGWG”), the Government of Japan hereby submits its initial input aiming to support the IGWG’s work to develop an outline of elements to be addressed in the PABS Annex. Our input is in the form of a diagram or chart as attached herewith in Page 2, capturing key elements provided for in Article 12 of the Pandemic Agreement. This input is without prejudice to Japan’s further inputs and proposals, and we reserve our right to make any revisions or new proposals subsequently during the IGWG process. The Government of Japan looks forward to working with other participants in IGWG process on developing an effective PABS system and the PABS Annex.
WHO Pandemic Agreement
Key structural issues to be addressed for the PABS System
To shape the structure of the PABS System, Japan proposes, in the first place, to focus on the basic elements, including those outlined here.
What needs to be shared?
Definitions of:
"Pathogen with pandemic potential"
"PABS Materials"
"PABS Sequence Information"
When should PABS Materials and Sequence Information be shared?
Pandemic Emergency/PHEIC/Outbreak/Inter-pandemic period
Trigger/Timing of sharing PABS Materials and Sequence Information
Who will be involved?
Definition of "participating manufacturer"
When should benefits be shared? Until when?
Where to store PABS Materials?
Where to register Sequence Information?
Whom to distribute"
(Criteria for identifying public health risks and needs?
What countries are in need?
How to share PABS Materials and Sequence Information with WHO?
When to conclude contracts?
(Prior to or during a pandemic?)
What are the benefits to be shared?
Vaccines, Therapeutics and Diagnostics
Monetary contributions
Additional benefits
How to operate/administer the PABS System?
Role of the Secretariat?
Relationship with other ABS instruments
Financing
MALAYSIA
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Malaysia.pdf
Submission of Text Proposal on the Annex on Pathogen Access and Benefits-Sharing (PABS Annex) of the WHO Pandemic Agreement
Submitted by: Government of Malaysia Date: [ 10 August 2025 ]
Note: this submission presents elements as requested, however it is without prejudice to any additional proposals on elements or textual suggestions as the discussion progresses.
SECTION 1: OBJECTIVE, SCOPE, AND USE OF TERMS
Article 1: Objective
To establish a safe, transparent and accountable multilateral WHO Pathogen Access and Benefit Sharing System pursuant to Article 12 of the Pandemic Agreement.
Article 2: Scope
The Annex applies to the rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information.
Article 3: Use of Terms
For the purposes of the Annex:
a. “Competent National Authorities (CNAs)” are government ministries, agencies, or other entities formally designated by each State, in accordance with its national laws and regulations, to exercise regulatory, supervisory, and enforcement functions with respect to the implementation of this Annex. CNAs shall be responsible for granting necessary authorizations, overseeing compliance with biosafety, biosecurity, and benefit-sharing requirements, coordinating with the National Focal Point (NFP) and the WHO, and ensuring that all activities involving PABS Materials and Sequence Information within their jurisdiction are conducted in conformity with this Annex, applicable international obligations, and domestic legal frameworks.
b. “Cybersecurity” refers to [placeholder]
c. “Cyberbiosecurity” refers to [placeholder]
d. “Data Access Agreements (DAAs)” refer to legally binding or standardized contractual instruments) from all users of database intending to access PABS Sequence Information established under the PABS System to govern the access, use, sharing, and management of PABS Sequence Information provided by Member States, laboratories, or other authorized entities. DAAs set out the rights, obligations, and conditions for recipients, including safeguards for data security, confidentiality, integrity, availability, and conditions relating to intellectual property; obligations to ensure rapid and transparent sharing of results; and mechanisms for equitable benefit-sharing.
e. “Digital Object Identifiers (DOIs)” are unique, persistent alphanumeric string assigned to a specific digital object, such as a dataset, sequence, publication, or other electronic record, which provides a permanent link to its location on the internet and enables reliable identification, citation, and tracking of the object over time, even if its underlying location or metadata changes.
f. “National Focal Point (NFPs)” are entities designated by each Provider State, in accordance with national laws and/or domestic laws, policies and regulations, to act as the primary authority responsible for authorizing, coordinating, and monitoring the release, transfer, and reporting of PABS Materials and Sequence Information. NFPs shall serve as the official liaison between the Provider State, WHO, and other relevant stakeholders, ensuring that all transfers and uses of PABS Materials and Sequence Information are conducted in compliance with the provisions of this Annex, applicable international obligations, and national legal requirements.
g. “Pathogen Access and Benefit Sharing System” refers to [placeholder]
h. “Pathogens with pandemic potential” is any pathogen that is novel (not yet characterized) or known (including a variant of a known pathogen), is highly transmissible human to human and can cause a pandemic emergency as defined in the IHR.
i. “PABS Materials and Sequence Information”
i. “PABS Materials” refers to live isolates of pathogens with pandemic potential, and parts thereof, modified pathogens, and any other materials derived from, generated or prepared using the PABS Materials.
ii. “PABS Sequence Information” shall include genetic, genomic, and proteomic data, including full or partial sequences, annotations, synthetic data, and digital sequence information (DSI), necessary for scientific utility.
j. “Participating manufacturer” means public or private entities including academic institutions, government owned or government subsidized entities, non-profit organizations or commercial entities that develop and/or produce vaccines, therapeutics and diagnostics for pathogens with pandemic potential and has entered into legally binding contracts with WHO for accessing PABS Material or Sequence Information.
k. “Standard Material Transfer Agreements (SMTAs)” are standardized, legally binding contracts adopted under the PABS System to regulate the transfer, receipt, and use of Pathogen Materials (including clinical specimens, isolates, and derivatives) between providing entities (such as Member States, laboratories, or biorepositories) and receiving parties (including manufacturers, research institutions, or public health agencies). SMTAs specify the rights and obligations of all parties, including conditions for use, restrictions on onward transfer, intellectual property considerations, requirements for transparency, and benefit-sharing commitments (monetary and non-monetary).
Article 4: Access to PABS System
1. Access to the PABS System shall be governed by Standard Material Transfer Agreements (SMTAs) and Data Access Agreements (DAAs). These agreements ensure traceability, transparency, biosafety and biosecurity, legal certainty, compliance with national laws and/or domestics laws and policies, and fair and equitable benefit-sharing. All access shall be guided by the principles of Prior Informed Consent (PIC) and Mutually Agreed Terms (MAT) to respect national sovereignty and ensure fairness. Signed SMTAs and DAAs imply PIC and MAT with respect to specific resources covered under PABS System for PABS purposes specified in those agreements. For PIC and MAT for any other use beyond those specified purposes, the recipients have to approach provider country’s competent national authority.
2. Each State Party shall designate National Focal Point (NFP) or other competent national authorities, as appropriate, to authorize the release and facilitate the transfer of PABS Materials and Sequence Information in accordance with the provisions of this Annex. Each State Party shall conduct risk assessment prior to any sharing of PABS Materials and Sequence Information.
3. Access to PABS System shall be subject to the following structure, whereby:
a. Nationally authorized laboratories forming a network coordinated by WHO to provide and access PABS Materials after signing SMTA1.
b. Entities/Persons outside this network of national authorized laboratories may seek access to PABS materials by signing an SMTA2 with WHO. Such SMTAs should specify PABS Materials, purposes and benefits arising therefrom.
c. Access to WHO sequence database shall be available to all registered users, on a non-discriminatory basis subject to acceptance of DAA.
4. WHO Collaborating Centres and designated National Reference Laboratories within a WHO coordinated laboratory network for sharing PABS Material and Sequence Information shall serve as the primary hubs for the receipt, storage, and redistribution of such materials. Sequence Information shall be deposited, managed, and disseminated exclusively through a WHO database, which shall maintain secure, transparent, and auditable systems for access and use, ensuring that such sharing is conducted in a manner that is consistent with the PABS system and traceable.
5. Only authorised national laboratories that agrees to accept the Terms of Reference (ToR), shall be authorized to share PABS Materials under this Annex.
6. PABS Sequence Information shall only be deposited in and accessed through WHO database, operating under governance rules designed to uphold transparency, equity, and the rights of State Parties. Such rules shall include, at a minimum:
a. Mechanisms to protect provider country rights, including acknowledgment and tracking of all downstream uses of the Sequence Information;
b. Mandatory user registration and acceptance of legally binding Data Access Agreement (DAA), including provisions on benefit-sharing obligations arising from the sharing/utilization of the Sequence Information; and
c. Digital traceability through the assignment of persistent identifiers, such as Digital Object Identifiers (DOIs), for each uploaded sequence to ensure accountability and auditable use. Users should be under an obligation not to remove such identifiers.
Article 5: Responsibility of WHO and COP
1. WHO under the guidance and authority of Conference of Parties to the WHO Pandemic Agreement shall provide the infrastructure for the PABS System, ensuring it is functional, transparent, and accessible to all Parties.
2. WHO shall be responsible for managing and maintaining global registries of PABS Materials and Sequence Information; coordinating the collection, allocation, and disbursement of monetary and non-monetary benefit-sharing contributions; and overseeing and verifying compliance with Standard Material Transfer Agreements (SMTAs), Data Access Agreements (DAAs), and benefit-sharing commitments.
3. WHO shall record and monitor all accesses, transfers and uses of PABS Materials and Sequence Information, tracking each item from its source through to final use and disposition. Each material sample and data entry shall be assigned a unique identifier, such as a barcode or Digital Object Identifier (DOI), to enable full traceability.
Article 6: Safe Transfer of PABS Materials
1. All transfers of PABS Materials shall be undertaken in strict compliance with biosafety and biosecurity protocols approved by the WHO, utilizing couriers certified to handle materials at Biosafety Level 2 or 3, as appropriate. Prior to any transfer, the transferring entity shall ensure the use of tamper-proof and secure packaging, and maintain a complete chain-of-custody record to guarantee the integrity and traceability of the materials. The provider and the WHO Collaborating Centre shall be notified, in a timely manner, of the receipt and final disposal of such materials to ensure transparency, oversight, and compliance with applicable provisions of this Annex.
2. All parties involved in the transfer, including the sender, transporter, and recipient, shall maintain adequate liability coverage to address any incidents, accidents, or damages that may arise during the transfer process.
3. WHO should cover the costs of shipment incurred by developing countries.
Article 7: Rapid and Timely Sharing of Benefits
1. Benefits arising from the use of PABS Materials and Sequence Information shall be shared without undue delay at three key stages:
a. At the onset of a disease outbreak having potential to become a pandemic emergency or Public Health Emergency of International Concern (PHEIC): Through the rapid deployment of vaccines, therapeutics, and diagnostics (VTD), accompanied by immediate financial and technical support to affected developing countries to ensure equitable access and timely response as well as licensing for diversification of manufacturing to developing countries, to address shortage of supplies;
b. At all times: regular building and management of national, regional or international stockpiles, and support for strengthening health systems, laboratory capacity and research and development infrastructure, and manufacturing readiness, particularly in developing countries; and
c. Post-pandemic period: Through sustained financial and other support to be identified aimed at building resilient health systems diversifying production and replenishing regional and global stockpiles of VTDs.
2. WHO shall coordinate and oversee the establishment of transparent timelines and mechanisms to track the provision, allocation, and delivery of such benefits, including VTDs, in accordance with this Annex.
3. All recipients of PABS Materials and Sequence Information shall agree to contribute to monetary and non-monetary benefits as detailed in the respective SMTAs and DAAs they sign, depending upon their type of use of PABS Materials and Sequence Information. SMTAs and DAAs should detail out operational aspects of these benefits, for instance, set-asides of VTDs to be shared during Pandemic Emergencies and PHEICs and triggers for such benefit sharing.
Article 8: Monetary Contributions to the PABS System
1. Monetary contributions to the PABS Fund shall be made by recipients of PABS Materials and Sequence Information engaged in generating revenue from the direct and indirect use of PABS Materials and Sequence Information, including laboratories, institutions, researchers, developers and manufacturers of vaccines, therapeutics, and diagnostics (VTDs). They shall commit to provide such contributions by signing SMTAs and DAAs.
2. Annual monetary contribution is based on the revenue generated from the commercialization of products and services, directly or indirectly, from using the PABS system, in accordance with rates and thresholds to be determined and set out in implementing arrangements.
3. Funds collected pursuant to this Article shall be allocated, in a transparent and equitable manner, based on public health risk and need, with priority to countries most severely affected by the pandemic, to developing countries lacking domestic manufacturing capacity, and to WHO-coordinated mechanisms for stockpiling, logistics, and the equitable global distribution of vaccines, therapeutics, and diagnostics (VTDs) and as further determined.
Article 9: Non-Monetary Contributions
1. Recipients of PABS Materials and Sequence Information shall, through legally binding contracts with WHO, commit to providing non-monetary contributions to support equitable access and pandemic preparedness, including:
a. In case they develop/produce VTDs:
[x]% of real-time production to WHO to respond to public health risks and events.
[Y]% of real-time production to WHO during PHEICs.
[10+10]% to WHO during pandemic emergencies as per Article 12.
Granting of non-exclusive licenses to developing country manufacturers through WHO.
b. In case of other users:
building new surveillance technologies – to provide affordable access to such technologies;
building new prediction softwares in epidemiology – to provide affordable access to such softwares;
universities that undertake cutting edge research – to provide partnerships and research collaborations etc.
2. Such contributions shall be monitored, enforced, and reported by WHO to ensure compliance with the provisions of this Annex and to facilitate equitable benefits haring across all participating States.
Article 10: Facilitation of Manufacture and Export
WHO and Member States shall take all necessary measures to facilitate the timely manufacture and global availability of vaccines, therapeutics, and diagnostics (VTDs) produced under the PABS System, including measures that will facilitate export clearances on faster and priority basis.
Article 11: Allocation and Distribution of VTDs
1. WHO shall determine allocation and distribution of VTDs, under guidance of COP, including through the Global Supply Chain and Logistics (GSCL) Network, to ensure that donated and purchased VTDs are distributed equitably based on public health need.
2. Decisions on the allocation and distribution of VTDs under this Article shall be guided by transparent and equitable criteria, including:
a. Epidemiological risk, taking into account the incidence, transmissibility, and severity of the pathogen of concern;
b. Economic vulnerability, with particular priority afforded to developing countries and other resource-limited or marginalized settings; and
c. Proximity to outbreaks and population density in affected or at-risk areas, to ensure timely and proportionate allocation based on public health need.
SECTION 3: GOVERNANCE OF THE PABS SYSTEM
Article 12: Governance Mechanisms
1. The PABS System shall operate under a multi-tiered governance structure designed to ensure transparency, equity, and accountability, comprising the following elements:
2. The Conference of the Parties (COP) to the WHO Pandemic Agreement shall establish a dedicated PABS Secretariat, which shall be responsible for managing and maintaining global registries of PABS Materials and Sequence Information; coordinating the collection, allocation, and disbursement of monetary and nonmonetary benefit-sharing contributions; and overseeing and verifying compliance with Standard Material Transfer Agreements (SMTAs), Data Access Agreements (DAAs), and benefit-sharing commitments. The PABS Secretariat shall exercise its functions in full respect of national laws, sovereignty, and applicable international obligations, working in partnership with the competent authorities of Provider States.
3. The Conference of the Parties (COP) to the WHO Pandemic Agreement shall inter alia approve strategic priorities, operational policies, and budgetary allocations for the PABS System; ensure the timely and equitable distribution of vaccines, therapeutics, and diagnostics (VTDs) based on public health risk and equity considerations; and review and take decisions on the annual PABS implementation and performance report prepared by the PABS Secretariat.
4. Day-to-day operations of the PABS System shall be coordinated and administered by the WHO, including conducted through WHO-designated laboratories, comprising national and regional reference laboratories responsible for the receipt, handling, and redistribution of PABS Materials; WHO global databases for the secure storage and dissemination of PABS Sequence Information; and distribution centres responsible for the storage and delivery of stockpiled or donated vaccines, therapeutics, and diagnostics (VTDs). All such operational laboratories shall, as a condition of designation and continued participation, comply with internationally recognized biosafety and biosecurity standards, transparency requirements, and reporting obligations as set forth in this Annex.
5. Under the authority of COP, WHO acting as PABS Secretariat, shall ensure the PABS System is functional, transparent, accountable to all Parties. WHO’s role will typically include:
a. Establish and maintain the central governance and coordination platform for the PABS System, including oversight of registries, data standards, and compliance mechanisms.
b. Act as the neutral intermediary between Provider (who share PABS Materials and Sequence Information) and Recipients (e.g., manufacturers, research institutions) to ensure equitable access and benefit-sharing.
c. Develop, host, and maintain the WHO PABS Sequence Information Database – a secure, standardized global database for PABS Sequence Information, accessible to Parties and registered users that have accepted DAAs.
d. Manage digital registries and traceability systems, including Digital Object Identifiers (DOIs) for samples and sequence information, to track material and sequence use and enforce benefit-sharing obligations.
e. Provide secure digital platforms for Standard Material Transfer Agreements (SMTAs) and Data Access Agreements (DAAs).
f. Coordinate WHO-led global stockpiling and distribution mechanisms for vaccines, diagnostics, and therapeutics (VTDs) during pandemic emergency or PHEICs and to prevent such occurrences.
g. Support rapid and secure shipment logistics for PABS Materials, especially for emergency response and R&D needs.
h. Provide training, infrastructure funding, and technical support to national laboratories, sequencing centres, and manufacturing hubs (particularly in developing countries).
i. Facilitate technology transfer and local production capacity-building to promote equitable access and self-reliance.
6. WHO shall establish and maintain a publicly accessible roster of all manufacturers participating in the PABS System. This roster shall include, at a minimum:
a. Information on each manufacturer’s production capacity for vaccines, therapeutics, and diagnostics (VTDs);
b. Details of their monetary and in-kind contributions, including dose donations and licensing arrangements; and
c. Records of their compliance with the obligations set forth in this Annex, including adherence to benefit-sharing, licensing, and reporting requirements.
7. The PABS System shall undergo independent and periodic monitoring and evaluation to assess the effectiveness and functionality of access and benefit-sharing mechanisms, the equity and timeliness of monetary and non-monetary benefit delivery, and the compliance of all participating manufacturers, laboratories, and databases with the obligations set forth in this Annex. Findings of such evaluations shall be compiled in public reports and shared with all WHO Member States to ensure transparency, accountability and continuous system improvement.
SMTAs and DAAs
Ensure compliance with all applicable biosafety and biosecurity standards, and fulfill mandatory reporting obligations, including the notification of the source, characteristics, and movements of PABS Materials and Sequence Information,
Cooperate fully with WHO, PABS Secretariat and the National Focal Point (NFP) to ensure the traceability of PABS Materials and Sequence Information, and to facilitate the monitoring, enforcement, including the use of registries, digital object identifiers (DOIs), and other mechanisms for tracking and verification.
Ensure full compliance with cyber biosecurity standards, and fulfil mandatory reporting obligations, including disclosure of utilization, and downstream development of PABS Materials and Sequence Information in accordance with the procedures established under the PABS System;
Cooperate fully with WHO to fulfill all monetary and non-monetary benefit sharing requirements as stipulated in Standard Material Transfer Agreements (SMTAs), Data Access Agreements (DAAs), or other legally binding instruments under the PABS System.
Submit summary reports to WHO detailing their use, transfer, and disposition of such materials and data as specified in SMTAs and DAA, including reports on analysis, risk assessment and other test results. Non-compliance with reporting or traceability obligations shall trigger enforcement measures as specified under this Annex.
Not be used in any activity that may lead to the development, or production of biological agents, toxins, weapons, equipment, or means of delivery specified in Biological Weapons Convention;
Acknowledge the contributions of the persons and institutions who submitted the PABS Sequence Information as well as the originating laboratories that first collected the clinical specimen and isolated the pathogen with pandemic potential;
Not seek or assert intellectual property over PABS Sequence Information and PABS Materials, or parts thereof, in any form, including any modified forms or for any use;
Share full regulatory data for accelerating regulatory approvals in at risk countries.
RUSSIAN FEDERATION
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Russian_Federation.pdf
Draft as of 10 July 2025
Structural elements of Article 12 of the WHO Pandemic Agreement
Note - In general, the paragraphs of Article 12 of the WHO Pandemic Agreement are represented at the section level for ease of cross-reference and transparency.
Section A – Scope (Article 12.1)
The rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes.
Section B bis - Biosafety, biosecurity, risk assessment, export control and data protection (Articles 12.1 and 12.5(e))
The key parameter for the effectiveness of the PABS System is to ensure that it operates in accordance with the highest requirements in the field of biosafety, biosecurity, risk assessment, export control and protection of data related to the exchange of pathogens with pandemic potential.
The PABS System provides for the distribution of pathogens with pandemic potential, which means that the distribution carried out by the PABS System may include threatening pathogens or high threat pathogens that are either already on the lists of states for export control or can be promptly added to such lists by them. In the event of the emergence of a pathogen with pandemic potential, the state (states) may promptly add it to the "List of Microorganisms..." subject to export control, and from the moment this decision comes into force, this microorganism and certain materials and technologies associated with it will become subject to export control requirements, including restrictions on its free transfer to third parties.
Biosafety, biosecurity and risk assessment
− Ensuring that recipient organizations of pathogens with pandemic potential have the necessary certified laboratory facilities, equipment, personnel, and have the appropriate national licenses and permits to carry out research work with the received pathogenic materials of the corresponding pathogenicity group.
− Laboratory work with and storage of received pathogens with pandemic potential should be carried out in strict compliance with national biosafety and biosecurity regulations and guidelines or in accordance with international regulations and guidelines (e.g. Laboratory Biosafety Manual, 4th edition. World Health Organization (2020)), if these are used as a basis for national regulations.
− In the absence of national biosafety and biosecurity regulations and certified laboratory facilities and personnel in the recipient country, pathogens with pandemic potential should not be transferred to such a country. This decision is made on the basis of a comprehensive risk assessment conducted by the country exporting pathogens with pandemic potential.
Export control
− Export control is a set of measures ensuring the implementation of the procedure established by national legislation for the implementation of foreign economic activity in relation to goods, information, works, services, results of intellectual activity (rights to them), which can be used in the creation of weapons of mass destruction, their delivery vehicles, other types of weapons and military equipment, or in the preparation and (or) commission of terrorist acts.
− The main objectives of export control are:
Protecting the interests of the country exporting controlled goods and technologies.
Implementation of the requirements of international agreements of the country-exporter of controlled goods and technologies in the area of nonproliferation of weapons of mass destruction, their means of delivery, as well as in the area of control over the export of military and dual-use products.
Counteracting international terrorism.
− Requirements and restrictions in the area of export control apply to controlled goods and technologies - raw materials, materials, equipment, scientific and technical information, works, services, results of intellectual activity (rights to them), which, due to their characteristics and properties, can make a significant contribution to the creation of weapons of mass destruction, their delivery vehicles, other types of weapons and military equipment, as well as products that are particularly dangerous in terms of the preparation and (or) commission of terrorist acts.
− In the context of Article 12 of the WHO Pandemic Agreement and this PABS Instrument (Annex), controlled goods include pathogens with pandemic potential that are included in the “Lists of Microorganisms, Toxins, Equipment and Technologies Subject to Export Control” adopted by countries that are party to the WHO Pandemic Agreement and countries that are party to the BTWC in accordance with their applicable national export control legislation.
In the event of transfer of pathogens with pandemic potential, that are not subject to export control, their exchange within the framework of the PABS System shall be carried out in accordance with other national legislation regulating the import and export of pathogenic microorganisms. −
The following restrictions apply to controlled pathogens with pandemic potential:
- Export from the exporting state to foreign states of controlled pathogens with pandemic potential is permitted for purposes not prohibited by the BTWC.
- Foreign economic transactions with controlled pathogens with pandemic potential, involving their transfer to foreign persons, are carried out on the basis of one-time or general licenses issued by the national agency responsible for export control.
- The decision to issue or refuse to issue a one-time license or permit is made on the basis of the results of the state examination of the foreign economic transaction, conducted in accordance with the established procedure by the national agency responsible for export control, together with other interested national agencies, as determined by national legislation in the field of export control.
- The agreement (contract, agreement) providing for the transfer of controlled pathogens with pandemic potential to a foreign party shall specify:
- the purpose and place of use of controlled pathogens with pandemic potential,
- end user of controlled pathogens with pandemic potential,
- obligations of a foreign person providing that the controlled pathogens with pandemic potential received by it:
will be used only for the stated purposes, not related to the creation of bacteriological (biological) weapons or the implementation of other activities prohibited by the BTWC;
will not be re-exported or transferred to anyone without the written permission of the participant in foreign economic activity from the exporting country, agreed upon with the national agency responsible for export control.
− If a foreign person is an intermediary, the obligations specified in subparagraphs a) and b) above shall also be assumed by the end user of controlled pathogens with pandemic potential, and the obligations may be drawn up in the form of a separate document.
− When transferring controlled pathogens with pandemic potential to states that are not parties to the BTWC, the obligations of the foreign person specified above in subparagraphs a) and b) must be confirmed by a document from the authorized body of the state in which the controlled pathogens with pandemic potential will be used.
− In the event of a violation by a foreign person of the obligations specified in subparagraphs a) and b) above, the national authority responsible for export control shall suspend the validity of issued and the issuance of new one-time licenses for the transfer of controlled pathogens with pandemic potential to that foreign person until the violation is corrected.
− Participants in foreign economic activity from the exporting country - licensees are obliged to immediately inform the national agency responsible for export control of the violation by a foreign person of the obligations provided for in paragraphs a) and b) above.
− A permit for re-export (transfer to a third party) of controlled pathogens with pandemic potential exported from the exporting country shall be issued to a foreign person by a participant in foreign economic activity from the exporting country in agreement with the national agency responsible for export control, or in accordance with another procedure established by national legislation in the field of export control.
− The decision to approve or refuse to approve re-export (transfer to a third party) is made by the national agency responsible for export control, based on the results of the state examination of the foreign economic transaction. The state examination of the foreign economic transaction is conducted in accordance with the established procedure by the national agency responsible for export control, together with other interested national bodies and agencies that previously participated in the state examination of the foreign economic transaction, based on the results of which a one-time license (permit) was issued to a participant in foreign economic activity from the exporting country.
Data protection
− Access to and protection of genetic sequence information on pathogens with pandemic potential contained in national genetic information databases shall be regulated in accordance with the national legislation of the countries that are party to the WHO Pandemic Agreement.
− Access to and protection of genetic sequence information on pathogens with pandemic potential contained in international genetic information databases shall be governed by the terms and conditions of such databases.
− In the absence in a country/territory of a national database of genetic information, including information on genetic sequences for pathogens with pandemic potential, access to such information shall be provided through the agreement on the terms of transfer between the country that is a party to the WHO Pandemic Agreement and the PABS System, taking into account the provisions of the PABS System.
Section B – Elements to be developed in the PABS System (Article 12.2 and 12.5)
(1) Definitions (Article 12.2) Pathogens with pandemic potential; (Article 12.2)
«Pathogen with pandemic potential» is a new (not yet characterized) or known (including variants) infectious agent (virus, bacterium or other microorganism) that possesses a set of biological characteristics that significantly increase its likelihood of causing a global outbreak of disease (pandemic)/PHEIC. These characteristics include:
High transmissibility - the ability to be transmitted with high efficiency from person to person (for example, by airborne droplets, contact, fecal-oral route, etc.) under conditions of normal social interaction.
Population vulnerability: the ability to infect people who lack significant preexisting immunity (natural or vaccine-derived), making a large proportion of the population susceptible.
Ability to spread widely: potential for rapid and uncontrolled spread across international borders using modern transportation networks and globalized societies.
Potential to cause high levels of morbidity and mortality: potential to cause severe disease leading to serious health consequences (prolonged hospitalization, disability, death), creating excessive financial and social burden on health systems and society as a whole.
Difficulties in control: lack or limited availability of effective and widely applicable countermeasures (specific vaccines, effective treatments in the early stages of an outbreak).
PABS Materials and Sequence Information; (Article 12.2)
«Materials and information on the genetic sequences of the PABS» – pathogens with pandemic potential in the form of samples from people or animals (in the case of zoonotic or zooanthroponotic pathogens) or in the form of isolated cultures and information on the genetic sequences of these pathogens, received by the PABS System in the established manner.
«Genetic sequence (information)» – a sequence of nucleotides in nucleic acid polymers.
«Genetic data» – information on the genetic information of various biological objects, presented in a form suitable for obtaining (collecting), systematizing, accumulating, storing, clarifying (updating, changing), using, distributing (including transferring) and destroying such information.
Participating manufacturer (Article 12.6(a))
Other technical terms
blank
Modalities, terms and conditions on access and benefit sharing that provide legal certainty (Article 12.5(b))
Legal nature (Article 12.2)
Coordination and operation of the PABS System, in collaboration with relevant international organizations and relevant stakeholders (Article 12.2)
Administration and coordination of the PABS System by WHO (Article 12.2)
Sharing of material and sequence information and of benefits, both monetary and nonmonetary, including annual monetary contributions and vaccines, therapeutics and diagnostics (Article 12.5(a))
Facilitation and acceleration of research and innovation (Article 12.5(c))
Complementarity with the PIP Framework and other relevant ABS instruments (Article 12.5(d)(i))
Review and alignment of national and/or regional ABS measures (Article 12.5(d)(ii)) National and/or regional access and benefit-sharing measures are not identical to and do not replace export control legislation for controlled pathogens with pandemic potential.
Consistency with applicable law related to risk assessment, biosafety, biosecurity and export control of pathogens, and data protections (Article 12.5(e))
Facilitation of manufacture and export of vaccines, therapeutics and diagnostics (Article 12.5(f))
Section C – Traceability and open access to data (Article 12.3)
The provision of pathogens with pandemic potential is carried out in accordance with international rules (BTWC) and national legislation in the field of export control, with priority given to national norms and rules (in the case of the transfer of controlled pathogens with pandemic potential) or other national legislation regulating the import and export of pathogenic microorganisms (in the case of the transfer of pathogens with pandemic potential that are not subject to export control).
The provision of information on the genetic sequences of PABS is carried out in accordance with international rules (in the case of international databases) and national legislation in relation to national databases of genetic information – with priority given to national norms and rules.
The free transfer of materials and information on the genetic sequences of the PABS is permitted only for scientific purposes. Commercial use of these materials and information requires a benefit-sharing agreement as defined in the PABS Instrument.
Section D – Consistency with Nagoya Protocol (Article 12.4)
The PABS instrument does not replace the Nagoya Protocol, but complements it in the event of pandemic emergencies and PHEICs, with the understanding that nothing in this paragraph creates obligations for States that are not parties to the Convention on Biological Diversity and/or the Nagoya Protocol.
Section E – Provisions in the event of pandemic emergency (Article 12.6)
Rapid access to real-time production of vaccines, therapeutics and diagnostics (Article 12.6(a))
Distribution of vaccines, therapeutics and diagnostics based on public health risks and needs, with particular attention to the need of developing countries (Article 12.65(b))
In distributing vaccines, medicines and diagnostics received by the PABS System, priority should be given to countries that have provided a pathogen with pandemic potential on the basis of or using which certain pandemic countermeasures have been developed. Further distribution of such resources is carried out depending on the level of risk to public health and public health needs, with particular attention to the needs of developing countries.
Section F – Provisions in the event of a public health emergency of international concern (Article 12.7)
In emergency situations involving the occurrence or risk of outbreaks of infectious diseases, in order to take measures to implement protective measures, a decision to issue a one-time license for the import of controlled pathogens with pandemic potential may be taken by the national agency responsible for export control, subject to the written consent of the federal executive body authorized to exercise functions for the control and supervision of infectious diseases.
Section G – Additional benefit-sharing provisions (Article 12.8)
Section H – Other elements for effective operationalization of the PABS System (Article 12.9)
The national agency responsible for export control, based on the results of the state examination of a foreign economic transaction, has the right to establish as a mandatory condition for the transfer of controlled pathogens with pandemic potential to a foreign person the acceptance by the end user of obligations to provide the participant in foreign economic activity from the exporting country with:
The right to verify the use of obtained controlled pathogens with pandemic potential.
Delivery confirmation certificate or other document issued by the authorized body of the state of end use, certifying the import of controlled pathogens with pandemic potential into the territory of its state.
SOUTH AFRICA
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/South_Africa.pdf
10 August 2025
Draft Outline and Elements for the Pathogen Access and Benefit Sharing System Submission made by South Africa to the Intergovernmental Working Group
This submission is without prejudice to any further elements and textual proposals and/or modifications that may be placed from time to time.
I. Institutional/Structural Elements of PABS
I.1. Conference of Parties
The Conference of Parties (COP) to the World Health Organisation (WHO) Pandemic Agreement is the ultimate decision-making authority with respect to the Pathogen Access and Benefit Sharing (PABS) system. WHO coordinates and administers the PABS System under the authority and guidance of the COP.
The COP adopts, amends and periodically reviews the instruments and agreements used to implement the PABS System, including SMTAs/DAAs, Technical Standards (e.g., lab safety, transport of biological materials, cyber-biosecurity and persistent identifiers), a Revenue Calculation Annex and a Procurement Policy. The COP may complement, amend or replace instruments to improve implementation.
For legal certainty, the PABS Instrument forms an integral part of the Agreement and shall not weaken essential elements, namely: scope including DSI/derivatives; conditional access via standard agreements with flow-down obligations; minimum non-monetary and monetary benefit-sharing floors; transparency; and compliance/enforcement. The COP establishes and oversees a PABS Equity Fund.
I.2. WHO Secretariat serving as PABS Secretariat [PIP Framework Model]
Just as in the case of the PIP framework model, the WHO Secretariat acts as the PABS Secretariat. The staffing and other resources are to scaled up stage by stage, taking into account the development and unfolding of functionalities within the PABS system, as well as the scale of transactions and the revenue available for such purposes.
Core operational functions include: (a) coordinating the WHO Laboratory Network and operating the WHO Repository for PABS Sequence Information (WHO PSI Repository) with a first-deposition rule and controlled mirroring; (b) maintaining public dashboards on setasides, deliveries, prices and monetary contributions, and a public roster of participating manufacturers; (c) administering standard SMTAs/DAAs and ensuring flow-down to affiliates and contractors; (d) implementing the Procurement Policy; (e) arranging shipment and last-mile logistics support for deliveries to developing countries as approved by the COP; (f) conducting independent/regular monitoring and evaluation and supporting an operational simulation exercise for continuous improvement;
I.3. WHO Laboratory Network accountable to COP
Currently, various WHO-coordinated networks are addressing different aspects of infectious pathogens. Some of these networks entail the sharing of clinical specimens and pathogens, subject to acceptance of specific Terms of Reference and principles that set out the specific purposes and uses of the biological materials by the receiving laboratory. They also accept terms and conditions contained in a material transfer agreement that contains the terms and conditions for the handling and use of such materials.
The GISRS network of laboratories under Pandemic Influenza Preparedness Framework (PIP Framework) use a “Standard Material Transfer Agreement (SMTA 1)”. They also have terms of reference and guiding principles that set out the specific tasks to be conducted with respect to PIP biological materials. Similarly, in the case of Biohub, Standard Material Transfer Agreements (SMTAs) govern the transfer of biological materials, accompanied by guiding principles. The SMTA has to be signed by the Provider, WHO Biohub facility and the WHO. In the case of WHO Coronavirus Network (CoViNet), to be a part of the reference laboratory, the laboratory has to meet certain criteria and accept specific Terms of Reference that list the various tasks of the laboratory and the uses of materials received.
From these approaches, and especially that of the PIP Framework, an instrument negotiated by Member States, a laboratory has to meet specific criteria, accept specific terms of reference detailing specific tasks and accept the terms and conditions that sets out the rights and responsibilities of the laboratories in the network, as providers and recipients in order to become part of WHO. At the same time dealing with pathogens, including transporting them outside the borders has serious implications for national sovereignty and security, and therefore only authorized laboratories become part of such international network.
Accordingly, it is proposed that a network of national laboratories authorised by their governments or by their Competent National Authority (CNA) be set up for the sharing of pathogens with pandemic potential (PPP), coordinated by WHO. To be a part of the WHOcoordinated laboratory network (WHO lab network), national authorised laboratories shall meet COP-adopted criteria, accept specific Terms of Reference detailing tasks, and execute the standard WHO-Lab SMTA (and the DAA where PSI access is sought) as a pre-condition to access, with flow-down to affiliates, contractors, CROs/CMOs and any downstream recipients (one-time execution unless a Party requires a signed agreement). PSI generated shall be deposited first in the WHO PSI Repository pursuant to the DAA; onward sharing within the Network is recorded in a COP-mandated tracking system using persistent identifiers.
Existing WHO networks or laboratories from networks may also become part of the WHO network, provided the laboratories have authorization from their respective governments. In doing this, establishment of a PABS system also provides an opportunity to streamline and standardise existing approaches that currently exist with respect to handling of pathogens with pandemic potential by laboratories coordinated by WHO.
The WHO lab network may also have laboratories with different capacities such as WHO Collaborating Centres and WHO Reference laboratories with specific terms of reference, although the same SMTA shall apply. It is important to ensure that each region and subregion has sufficient laboratory capacity to undertake the needed assessments and activities for prevention, preparedness and response of a health emergency. The COP and Secretariat shall support regional capacity development and periodic operational simulations to validate timelines, logistics and data workflows.
I.4. WHO PSI Repository and other authorised repositories (databases) accountable to COP
Another crucial issue in the PABS system is the sharing of PABS Sequence Information (PSI) generated from PABS Materials. Under the Convention on Biological Diversity and the Nagoya Protocol on Access and Benefit Sharing, sequence information generated from the genetic materials shall be regulated by the respective national legislations. There is also recognition of the need to share fair and equitable benefit sharing arising from the use of sequences.
In the case of the PABS system, there are five important pressing requirements: (i) to ensure that there is transparency and accountability to Parties providing the PSI and thus to the COP; (ii) to ensure PSI is made accessible without any form of discrimination, consistent with eligibility conditions and data-protection requirements; (iii) entities or persons accessing PSI have to agree (prior to access), to identify themselves and to use the PSI based on terms and conditions set out including to provide fair and equitable benefit-sharing; (iv) PSI remains comprehensively accessible via a WHO-designated point of first deposition with controlled mirroring (WHO PSI repository) and (v) to ensure PSI is not used in violation of national ABS laws, PABS instruments or for purposes beyond the PABS system, including by prohibiting removal of provenance tags and re-upload to non-authorised repositories.
To achieve these objectives, it is essential to establish a WHO PSI Repository and authorise other repositories or databases to host and disseminate PSI, under contracts with WHO and accountable to the COP.
The WHO PSI Repository shall ensure that PSI generated from PABS Materials is shared through a single WHO-designated point of first deposition and made accessible in accordance with COP-adopted instruments, rather than at the discretion of individual repositories or databases. Accessibility is provided through registered user accounts subject to a COP-approved Data Access Agreement (DAA). The WHO PSI Repository and authorised repositories shall implement certain requirements in particular user registration, acceptance of the DAA (See III.3 below) as well as ensure the PSI is sufficiently labelled as PABS sequence information, using persistent unique identifiers (e.g., DOIs), which shall be tracked via audit logs.
Authorised repositories or databases may mirror PSI from the WHO PSI Repository only under WHO contracts that enforce the DAA, preserve provenance tagging, prohibit detagging and re-upload to non-authorised databases, and ensure interoperability via WHO brokered connectors to external registries (e.g., IP, regulatory and clinical-trial registries). The COP shall adopt the mandatory fields with respect to the metadata, including any provenance data such as origin or source etc. mentioned above and minimum cyber biosecurity controls and audit requirements;
I.5. Advisory Group
Advisory group comprising experts from Parties (fair representation from developed and developing countries) shall be established to advise WHO regarding the operations of the PABS System and make recommendations for decision-making by the COP. The Advisory Group shall have a mix of relevant expertise and operate in an open and transparent manner. Members shall disclose and manage conflicts of interest; meetings and recommendations shall be published. Terms of reference on the functions of the advisory group shall be established by the COP.
To align with operational needs, the Advisory Group shall: (a) review and advise on technical standards for PSI stewardship (first deposition, controlled mirroring, persistent identifiers, cyber-biosecurity); (b) review and recommend updates to SMTAs/DAAs and to the Procurement Policy; (c) review and advise on metadata schemas and interoperability connectors; and (d) review findings from independent M&E and operational simulations, making recommendations to the COP.
II. Definitions and The Scope
II.1. Definition of PABS Materials
The scope of PABS System as per Article 12 of WHO PA shall remain pathogens of pandemic potential i.e. pathogens that can cause a pandemic emergency. Further criteria for determination of pathogen with pandemic potential may be defined by COP.
PABS Materials means isolates of PPP, parts thereof, and any materials derived from, generated or prepared using such pathogens or their parts, including attenuated strains, vectors, cell lines, reference standards, and synthetic or computationally-generated constructs arising from PABS inputs, shared by laboratories authorised by the Competent National Authority (CNA) within the WHO Laboratory Network.
If clinical specimens are being shared, their use is limited to isolation of PPP necessary for public-health response covered under the SMTA. Any other use requires prior written consent of the Provider Party’s CNA, and compliance with national law, ethics approvals and privacy protections.
II.2. Definition of PABS Sequence Information
PABS Sequence Information means digital sequence information and related data derived from PABS Materials or PSI, including information generated from the PABS Materials by using sequencing technologies, such as nucleotide (DNA/RNA) and amino-acid sequences, multi-omics (transcriptomic/proteomic), annotations, variant calls, consensus sequences, design files and associated metadata (including provenance), together with any computational outputs based on PABS inputs. Access to PSI is non-discriminatory but conditional on registration and execution of a Data Access Agreement (DAA); PSI is subject to first deposition in the WHO PSI Repository, controlled mirroring under WHO contracts, use of persistent identifiers (e.g., DOIs), and prohibitions on de-tagging and re-upload to non-authorised repositories.
II.3. Other definitions necessary for operational certainty inter alia
“Competent National Authority (CNA)” – the national authority designated to implement PABS, authorise participation in the WHO Laboratory Network, and liaise with WHO.
“Data Access Agreement (DAA)” – the COP-approved agreement governing access to and use of PSI, including user identification, traceability, preservation of provenance tags, flowdown obligations and benefit-sharing.
“Digital Object Identifier (DOI)” – a globally unique persistent identifier assigned to PSI records to enable interoperable traceability across repositories and registries.
“Cyber-biosecurity” – technical and organisational measures to protect the integrity, confidentiality and availability of biological data/systems; minimum controls adopted by the COP.
“Vaccines, therapeutics, and diagnostics for the pathogen causing the pandemic emergency,” includes repositioned medicines, and related ancillary supplies used for prevention, preparedness and response
Clarify ‘Participating manufacturer’: include any entity that accesses or benefits from PABS inputs, directly or indirectly (affiliates, CROs/CMOs, licensees) involved in research, development or manufacturing VTDs, to close the “voluntary” loophole.
II.4. Scope of the PABS system
The scope of the PABS system covers PPP and their PABS Materials and sequence information as defined above.
PABS Materials and PSI are governed under the PABS system for the specific purposes set out in the standard agreements SMTA/DAA, i.e. for pandemic prevention, preparedness and response. Access is conditional on execution of the applicable SMTA/DAA with flow-down to affiliates, contractors, CROs/CMOs and downstream recipients (“no access without obligations”), and on compliance with first deposition, traceability and transparency requirements. Minimum benefit-sharing floors (real-time set-asides and revenue-linked contributions) apply as provided elsewhere in this submission. For any purposes beyond PABS or outside the specified scope, national laws and regulations of the Provider Party shall apply.
III. Access to PABS Materials and Sequence Information
III.1. Transfer of PABS Materials with and within WHO Lab Network
III.1.1. Events Triggering Transfers
Nationally authorised laboratory within the WHO Lab Network may share PABS Materials with the WHO Lab Network, with immediate notification to the Provider CNA and the WHO Secretariat, based on its risk assessment of a disease causing pathogen as to whether such pathogen is of pandemic potential as defined.
III.1.2. Legally Binding Obligations applicable to transfer of PABS Materials with and within WHO Lab Network and Benefit-Sharing (signing of WHO-Lab Network SMTA required once)
The transfer of PABS Materials to a laboratory within the WHO Lab Network is subject to the standard WHO-Lab SMTA terms and conditions accepted upon enrollment and as updated by the COP/WHO. Execution of the WHO-Lab SMTA (and, where PSI access is sought, the DAA) is a pre-condition to access and applies with flow-down to affiliates, contractors, CROs/CMOs and any downstream recipients.
Compliance of the WHO Lab Network with the SMTA shall be monitored by the DirectorGeneral. In the event of non-compliance with the SMTA and the relevant ToR, appropriate action will be taken, including suspension or revocation of the WHO designation from the laboratory.
The WHO-Lab SMTA shall contain legally binding provisions including the following:
compliance with the tasks and activities set out in the laboratory ToR; uses beyond the ToR are prohibited and the national laws of the Provider Country shall apply in such cases.
benefit-sharing appropriate to laboratory functions, including non-monetary benefits (e.g., access to results, reference materials, training and technology packages) for Provider Countries, and cooperation to enable downstream monetary benefit-sharing where commercialisation occurs through the PABS Equity Fund.
clarity that any further sharing of PABS Materials within the Network is subject to the same terms and conditions as applicable to WHO Lab Network and recorded in the tracking system described below.
commitment not to share PABS Materials outside of the Network, unless the prospective recipient has signed the applicable material transfer agreement and, where PSI access is sought, the DAA; no onward transfer without flow-down of obligations.
commitment to share sequence information only through the WHO PSI Repository with first deposition in that repository; agreement to comply with the Data Access Agreement (DAA) (see element III.3), including preservation of provenance tags, prohibition of detagging and re-upload to non-authorised databases, and use of persistent identifiers (e.g., DOIs) for traceability.
commitments with regard to intellectual property i.e. that there will be no IP claims on the shared materials, or on any other modified forms or uses of the materials as well as any of the outcomes
commitment to record the receipt and any onward transfer of the PABS Materials in a tracking mechanism to be established. The tracking system shall use persistent identifiers and audit logs, and be interoperable with WHO-brokered connectors to external registries, as applicable.
to abide by any additional requirements of the PABS system, including WHO cyberbiosecurity and biosafety standards, reporting obligations and audit rights as adopted by the COP.
III.2. Transfer of PABS Materials to entities outside the Network
III.2.1. All prospective recipients (persons/entities) seeking access to PABS Materials, including for research and/or for development, manufacture, authorisation or supply of vaccines, therapeutics and diagnostics (VTDs) shall execute a WHO Standard Material Transfer Agreement (SMTA) approved by the COP as a pre-condition to access (“no access without obligations”). The SMTA flows down to affiliates, contractors, CROs/CMOs, collaborators and sublicensees. Obligations cover handling and use of PABS Materials and PSI, traceability, transparency and benefit-sharing arising from sharing/utilisation of PABS inputs.
The Standard Template for the Material Transfer Agreement shall, at a minimum, contain legally binding provisions on:
permitted specific uses of PABS Materials and PSI. These uses shall be only for pandemic prevention, preparedness and response (PPR) purposes. Uses beyond the SMTA scope require prior written authorisation by the Provider Country (via CNA) and compliance with national law, ethics and privacy requirements;
prohibition of onward transfer outside the PABS system without mandatory flow-down: no transfer to partners, collaborators, CROs/CMOs or any third party unless such party is registered and has executed the applicable SMTA (and, where applicable, DAA); all onward transfers are recorded in the COP-mandated tracking system;
commitment to generate and share PSI with first deposition in the WHO PSI Repository, with controlled mirroring only under WHO contracts; compliance with the DAA, preservation of provenance tags, use of persistent identifiers (e.g., DOIs), and a prohibition on de-tagging or re-upload to non-authorised repositories;
commitments with regard to intellectual property i.e. that there will be no IP claims on the shared materials, or on any other modified forms or uses of the materials as well as on any of the outcomes.
biosafety, biosecurity and cyber-biosecurity: prohibition/regulation of research of concern (e.g., enhanced PPP/GoF/DURC) consistent with WHO guidance and national law; prior review/authorisation where applicable; adherence to COP-adopted technical standards.
▪ Benefit Sharing obligations of the participating manufacturer:
(i) Annual Monetary contributions to the PABS Equity Fund calculated on global revenues from PABS-enabled products/services: [XX%] during a PHEIC/Pandemic and [YY%] interpandemic, as defined in the Revenue Calculation Annex;
(ii) 20% or more set aside of real-time production for WHO allocation by public-health need (with at least 10% donation and the balance at affordable prices) during pandemic emergency, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation;
(iii) 15% or more set aside of real time production for WHO allocation by public-health need (with at least half as donation and the balance at affordable prices) during PHIEC, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation
(iv) Supply to WHO stockpiles and outbreak response on request; WHO may arrange and, where appropriate, cover shipment and last-mile logistics for deliveries to developing countries;
(v) Pandemic emergency and PHEIC set-asides may be updated consistent with Article 12 and COP decisions; floors may be raised by COP but not lowered;
(vi) Licence to WHO (worldwide, non-exclusive, royalty-free) with sub-licensing to developing countries manufacturers covering background/foreground IP, regulatory data waivers/sharing, cell lines/seeds, process parameters and tacit know-how; time-bound transfers (30-day package; 60–90-day on-site support);
▪ Benefit Sharing obligations of all other recipients
(i) Annual Monetary contributions: where revenues arise from PABS-enabled services/tools, apply the inter-pandemic rate above; otherwise, provide non-monetary benefits (reference materials, training, method transfer, data/tools) as specified by COP;
(ii) Other fit-for-purpose benefits according to recipient type (e.g., sharing validated methods, QC panels, analytics scripts), as well as cooperation to enable downstream monetary benefit-sharing where commercialisation later occurs;
▪ Other contractual terms including duration of the agreement, termination, dispute resolution etc.; transparency (publication of SMTA; public dashboards on PABS usage, benefit sharing, deliveries and contributions); monitoring and implementation, consistent with national law and COP-adopted policies.
III.3. Access to PABS Sequence Information
III.3.1. User Registration and Data Access Agreement (DAA) signed as a part of Registration, as a pre-condition to access.
WHO PSI Repository and other authorised databases/repositories provide access only to users with verified identities, who have registered and have signed the DAA, as part of registration. Access is non-discriminatory but conditional on eligibility, user identification, and acceptance of COP-approved terms (including flow-down to affiliates, contractors, CROs/CMOs and downstream recipients), and on compliance with minimum cyber biosecurity controls and audit requirements.
III.3.2. The DAA is legally binding between WHO and each PSI user and commits users to at least the following terms:
PSI may be used only for specified PPR purposes; any other use requires prior written authorisation of the Provider Country (via CNA/NFP) and compliance with applicable national law, ethics and privacy protections.
commitments with regard to intellectual property i.e. that no IP to be claimed on the sequence information in any form and use as well as on the outcomes of the research activities.
biosafety, biosecurity and cyber-biosecurity requirements, including prohibition/regulation of research of concern (e.g., enhanced PPP/GoF/DURC) consistent with WHO guidance and national law; prior review/authorisation where applicable.
commitment with regard to persistent unique identifiers including the use of persistent identifiers (e.g., DOIs), preservation of provenance metadata/tags, and a prohibition on removal or alteration of such identifiers;
no onward sharing of PSI with any person or entity that has not executed a DAA and been registered by WHO; all onward access is recorded in repository audit logs;
commitment not to upload PSI to non-authorised repositories and not to re-upload mirrored PSI; interoperability with external registries (e.g., IP, regulatory and clinical-trial) shall occur only via WHO-brokered connectors that preserve PABS tagging and obligations;
III.4. Benefit Sharing obligations of the participating manufacturer, accessing PSI:
(i) Annual Monetary contributions to the PABS Equity Fund based on global revenues from PABS-enabled products/services: [XX%] during a PHEIC/Pandemic and [YY%] interpandemic, as defined in the Revenue Calculation Annex;
(ii) 20% or more set aside of real-time production for WHO allocation by public-health need (with at least 10% donation and the balance at affordable prices), during pandemic emergencies, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation;
(iii) 15% or more set aside of real-time production for WHO allocation by public-health need (with at least half as donation and the balance at affordable prices) during PHIEC, with delivery commencing ≤ 30 days after WHO EUL or first national authorisation
(iv) Supply to WHO stockpiles and outbreak response on request; WHO may arrange and, where appropriate, cover shipment and last-mile logistics for deliveries to developing countries;
(v) Pandemic emergency set-asides may be updated consistent with Article 12 and COP decisions; floors may be raised by COP but not lowered;
(vi) Licence to WHO (worldwide, non-exclusive, royalty-free) with sub-licensing to designated developing countries manufacturers covering background/foreground IP, regulatory data waivers/sharing, cell lines/seeds, process parameters and tacit know-how; time-bound transfers (30-day package; 60–90-day on-site support);
III.5. Benefit Sharing obligations of all other persons/entities accessing PSI:
(i) Annual Monetary contributions: where revenues arise from PABS-enabled services/tools, apply the inter-pandemic rate above; otherwise, provide non-monetary benefits (reference materials, training, method transfer, data/tools) as specified by COP;
(ii) Other fit-for-purpose benefits according to recipient type (e.g., sharing validated methods, QC panels, analytics scripts), as well as cooperation to enable downstream monetary benefit-sharing where commercialisation later occurs;
▪ Other contractual terms including duration, termination, dispute resolution, transparency (publication of DAA; public dashboards on PSI use, monitoring and implementation, consistent with national law and COP-adopted policies
IV. Fair and Equitable Sharing of Benefits
IV.1. All recipients of PABS Materials and/or PSI that generate revenue (directly or indirectly) from PABS-enabled products or services shall contribute annual monetary benefit-sharing to the PABS Equity Fund, calculated on global revenues in accordance with a COP-adopted Revenue Calculation Annex. Rates shall be [XX%] during a PHEIC/Pandemic and [YY%] inter-pandemic, as applicable. Recipients shall report revenues to WHO (with audit-light verification).
IV.2. All recipients shall provide non-monetary benefits appropriate to their role, in addition to monetary contributions. Manufacturers accessing PABS Materials and/or PSI shall, as a condition of the SMTA/DAA, provide at minimum:
(a) set-asides of real-time pandemic emergencies and PHEICs;
(b) support to WHO stockpiles and outbreak response;
(c) licences to WHO with sub-licensing to designated developing countries manufacturers, including transfer of cell lines/seeds, regulatory data, process parameters and tacit knowhow with time-bound milestones; and
(d) training, method transfer and access to reference standards/QC panels.
Non-manufacturing recipients (e.g., academic labs, repositories, service providers) shall provide fit-for-purpose non-monetary benefits (e.g., validated methods, analytics scripts, data tools, training) and cooperate to enable downstream monetary benefit-sharing where later commercialisation occurs. All obligations flow down to affiliates, contractors, CROs/CMOs and sublicensees and are recorded in COP-mandated tracking systems.
IV.3. The distribution and use of monetary and non-monetary benefits shall occur under COP direction, administered by the WHO Secretariat. The COP establishes allocation principles prioritising public-health needs with particular attention to developing countries, regional balance and surge capacity.
IV.4. PABS Compliance should be taken into account when Parties promote or incentivize R&D activities or manufacturing.
V. Accountability
The governance chain—from COP to the WHO Secretariat, and Advisory Group, to providers, repositories, laboratories and participating manufacturers—shall be established and published, including roles, reporting lines and escalation paths. Any issues of non-performance of obligations, and infringements of rights, should be addressed promptly through various dispute settlement processes, including negotiations, mediations and arbitration. Pending disputes settlement processes should not hinder the delivery of committed VTDs during the time of outbreaks or emergencies.
VI. Transparency
Mechanisms are established to ensure full transparency in the functioning of the PABS system. This includes for:
recording all transfers of PABS Materials as well as clinical specimens;
identifying and making available the list of registered users of PABS Sequence Information;
making available all signed SMTAs especially with entities outside the WHO Lab Network
making available all information with respect to benefit sharing received.
SWITZERLAND
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Switzerland.pdf
Federal Department of Home Affairs FDHA Federal Office of Public Health FOPH Division of International Affairs
Submission by Switzerland 6 August 2025
Structured List of Questions to be Addressed by the IGWG for Focused Discussions on the PABS Mechanism
Following the adoption of the WHO Pandemic Agreement by the World Health Assembly (WHA) in May 2025, Member States are now engaged in the negotiation of the annex establishing the Pathogen Access and Benefit-Sharing (PABS) System. In view of the initial commitment to finalize the annex by WHA79 in May 2026, Switzerland would like to support the Bureau and Member States in this process by proposing a structured list of questions to be addressed and the organization of a simulation exercise. The questions stem from Switzerland’s efforts to visualize how a future PABS System could be operationalized, based on the text (Art. 12) adopted by the WHA on 20 May 2025. The flow chart included on the last page of this document provides an illustration of this conceptual framework which aims to ensure that all important elements to be considered are taken into account. Furthermore, Switzerland proposes to organize a simulation exercise in collaboration with the WHO Collaborating Centre at the Spiez Laboratory, which we believe will meaningfully support Member States in further developing the annex.
1. Structured list of questions to be addressed
The questions below, stemming from the flowchart on the last page of this document, are both related to definitions and processes, the governance of the PABS System as well as the functioning of the access and benefit sharing of pathogens with pandemic potential. Grouped into four thematic areas, the list aims to support focused and constructive discussions, and to contribute to the coherent and effective development of the PABS Mechanism and its associated elements. In this regard, these questions would need to be discussed at different stages of the PABS negotiation process:
1. Cross-Cutting Issues
Definitions o “PABS Material”
“Pathogens with Pandemic Potential”
“Participating Manufacturer”
Interaction with Other Instruments or Mechanisms
How can overlap and duplication be avoided? (Art. 12.5.d)
What is the relationship with:
The PIP Framework?
The Nagoya Protocol?
National or regional ABS measures?
Agreements and Contracts
Will there be standardized contracts?
Could existing models serve as references (e.g., SMTA 1, SMTA 3)?
What elements are essential in the contracts?
2. Governance
Role of WHO
What is WHO’s role in the governance of the PABS System?
What is the mandate of the coordinating and administrative body under Art. 12.2?
Which departments or existing WHO mechanisms are implicated?
Is WHO financially and structurally equipped to fulfill these tasks?
Operationalization of the System
Who are the eligible participants (e.g., states, laboratories, universities, private sector, WHO)?
How will simultaneous implementation of all system elements be ensured? (Art. 12.2)
Will a compliance or oversight mechanism be established? Where?
What are the consequences of non-compliance?
Financing
How will the mechanism be financed in the long term?
- In “peace time”?
- During a “public health crisis”?
What role does the Coordinating Financing Mechanism play (Art. 18)?
What do the “annual monetary contributions” cover (Art. 12.5.a)?
Role of the Conference of the Parties (COP)
What is the COP’s role in governance?
Will there be reporting obligations?
Will monitoring and evaluation mechanisms be established?
How will the COP coordinate with WHO?
3. Access
Access Governance & Procedures
Who decides if and when access is granted?
What data will be shared (“PABS Materials”)?
How will sensitive data be protected?
Are States Parties obligated to share pathogens in certain situations (e.g., PHEIC)?
Can only Parties share pathogens? What happens if relevant pathogens are found in non-Parties?
How will the sovereignty over biological resources be respected?
Who bears the costs of collection, storage, sequencing, and sharing?
Channels and Infrastructure
What channels will be used to share material:
- Existing WHO laboratories (e.g., BioHub)?
How quickly must material be shared?
Can this system function effectively during crises?
Will training be provided to increase the number of BSL-4 labs globally?
4. Benefit-Sharing
Definition and Scope
What is the scope and definition of “benefits”
What is the scope of the different types of benefits?
- “set asides”
- “real time production”
- “donation”
- “affordable prices”
Optional benefits (Art. 12.8), including:
- Capacity building and technical assistance
- Research and development collaboration
- Licensing of technologies to manufacturers in developing countries
- Other forms of technology transfer
What is the scope of “medical countermeasures” in the PABS Mechanism?
- Is it limited to novel countermeasures? What about repositioned medications?
Triggers and Timing
What triggers the sharing of benefits?
Distribution and Delivery
What are the conditions and criteria for distribution of medical countermeasures?
What legal frameworks govern the delivery and distribution of medical countermeasures?
What is the role of WHO and national/regional authorities in regulatory approval?
How will legal risks related to novel pandemic vaccines be managed? (WHA78.1, OP15.10)
2. Proposal for a simulation exercise
Switzerland, in collaboration with the WHO Collaborating Centre at the Spiez Laboratory, proposes to organize a simulation exercise to support the negotiations of the annex. This simulation exercise will ideally be co-hosted by a Member State from the Global South and coordinated by the WHO with the WHO Biohub.
We suggest holding the simulation exercise between the third and fourth meetings of the IGWG, as a one-day event on the premises of the Spiez Laboratory in Switzerland.
Objective
This simulation exercise aims to test the practical feasibility and operational functionality of a potential PABS Mechanism by simulating a realistic pandemic emergency scenario. The focus is on exploring the existing draft and on identifying procedural, legal, and political challenges that may arise during the implementation of the system in a time-sensitive context. The key objectives include:
Assess how quickly and effectively pathogens and/or sequence data can be shared under the PABS framework.
Evaluate how benefit-sharing obligations are triggered and implemented.
Identify bottlenecks, ambiguities, and coordination challenges.
Generate insights to inform negotiation and design of the PABS mechanism.
The outcome of the exercise will be a report summarizing the findings and lessons learned, intended to support Member States in the further negotiation and refinement of the annex.
Exercise Format
We propose a tabletop simulation, similar to a role play, based on the available draft of the PABS Annex and on the adopted text of the Pandemic Agreement. The Exercise will simulate real-time decisions and processes that States Parties, WHO, Reference Laboratories, participating manufacturers and stakeholders would face when responding to an emerging disease threat related to a pathogen with pandemic potential.
Participants
To ensure a comprehensive discussion, we propose involving stakeholders across the PABS process. Alongside Member States, WHO, and the WHO BioHub, we foresee including industry partners, and civil society.
Switzerland welcomes the opportunity to work collaboratively with Member States, WHO, and relevant stakeholders to ensure the relevance of this simulation exercise.
TUNISIA
https://apps.who.int/gb/igwg/pdf_files/IGWG2-initial-text-proposals/Tunisia.pdf
The text below was machine translated from the French version.
Tunisian Draft Proposal for the PABS System Annex
Section A: Objective and Scope (Section A – Article 12.1)
This annex aims to specify the operational provisions of the PABS system in order to guarantee
Rapid, equitable, and traceable access to pathogens with pandemic potential and their genetic sequences;
Fair, transparent, and binding sharing of monetary and non-monetary benefits resulting from their use;
Protection of the sovereign rights of countries of origin, in accordance with the Nagoya Protocol.
It applies to all stakeholders involved in the transfer, analysis, exploitation and distribution of these biological resources, including commercial entities.
Section B: Elements to be developed in the PABS system:
I. Definitions (Section B.1 – Article 12.2):
PABS Materials and Sequence Information: “WHO Pathogen Access and Benefit-sharing Scheme” means any biological material (isolates, strains, clinical samples) and genetic data associated with a pathogen identified as having pandemic potential.
Pandemic Pathogen: Any biological agent that could cause rapid international spread with major public health impacts.
Participating Manufacturer: Any pharmaceutical or industrial entity that has access to a pathogen through the PABS.
Relevant International Organizations: Includes multilateral institutions with mandates in the areas of public health, research, biosafety, intellectual property, and health development financing.
Pecuniary Benefits: Financial benefits, including monetary contributions to the PABS Multilateral Fund, user fees, or any payments agreed upon in the SMTAs related to the use of shared resources.
Non-monetary benefits: Non-financial advantages, such as equitable access to health products, technology transfer, participation in research, training, and open sharing of scientific data and sequences.
In-kind benefits: Direct provision of health products (vaccines, treatments, diagnostics) to supplier countries, free of charge or at reduced cost, particularly in the event of a public health emergency.
II. Access Terms and Sharing Conditions (Section B.2, B.6)
All transfers of pathogens must be made via a signed SMTA (Standard Material Transfer Agreement), specifying:
Authorized uses (public health only);
Transfer schedule, volume transferred
Terms of use (scope and details of use, purposes, etc.)
Obligation to provide feedback (results, sequencing data, use)
Commitment and terms of benefit sharing in the event of exploitation (vaccines, treatment and diagnostic products, health products, patents, etc.).
Obligation to transfer technologies
Protection against deliberate misuse (storage and secure access)
Post-pandemic use
States Parties will maintain a national registry interconnected with the WHO and the designated platform.
III. Legal nature of the annex:
As a Party fully committed to the responsible governance of pathogens with pandemic potential, the Republic of Tunisia reaffirms the importance of ensuring that the obligations relating to the PABS system, including those set out in its annex, have legal force equivalent to those of the main treaty.
Tunisia recommends that:
The PABS Annex be explicitly designated as an integral, legally binding part of the treaty adopted under Article 19 of the WHO Constitution;
Any amendment to this Annex be subject to formal approval by States Parties, through a transparent and equitable governance process;
The Annex contain strong provisions on traceability, benefit sharing, transfer conditions (SMTAs), and technological cooperation, to ensure a fair balance between access to pathogens and effective health benefits for contributing countries.
Such a legal architecture is essential to build trust between Parties, prevent biopiracy, and ensure a fair, coordinated, and sovereign global response to future pandemics.
IV. Coordination and Operation of the PABS System in Collaboration with Relevant International Organizations and Stakeholders (Art. 12-2)
Collaboration must not be vague or unbalanced. It must be:
Inclusive
Geographically and functionally balanced
Under clear supervision of the States Parties
And transparent
The PABS system is coordinated by WHO, in collaboration with relevant international organizations and stakeholders, in accordance with their respective mandates.
Partner international organizations are selected based on objective criteria defined by a PABS Governance Committee and operate in the areas of public health, research, funding, intellectual property, or data sharing.
Any formalized collaboration between WHO and an external party must be made public, documented, and subject to compliance review by State Parties.
Operational platforms may be decentralized and entrusted to national or regional institutions, subject to compliance with PABS standards.
V. Coordination and Administration of the PABS System By the WHO
The Parties recognize the World Health Organization (WHO) as the central coordinating authority of the PABS system, responsible for its operational management, monitoring, strategic oversight, and support to the Parties, in accordance with the principles of transparency, equity, inclusive participation, and accountability.
Tunisia recommends that the WHO establish a dedicated governance body for the PABS system, composed in a balanced and multi-stakeholder manner. This body includes representatives from each WHO region, with equal representation, with a rotating chair alternating between high-income countries and low- and middle-income countries. It includes equitable representation from pathogen-supplying and user countries, as well as independent experts in ethics, human rights, biosafety, equity, and open science. This body is responsible for reviewing SMTAs, approving designated platforms, overseeing the implementation of benefit sharing, and recommending any necessary corrective measures.
An independent arbitration mechanism is established to resolve disputes related to the implementation of the PABS system. It is composed of experts appointed by the States Parties. This mechanism may be consulted in the event of disputes relating to access to pathogen resources, breached contractual commitments, or non-fulfilment of equitable benefit-sharing obligations.
The WHO ensures full transparency in the administration of the PABS system. All decisions related to approvals, access, distribution, or contractual commitments are made public via an online platform. Commitments made by private entities under the SMTAs are also published, including those relating to the production, distribution, price, and quotas of derived health products.
The WHO-led coordination includes an active mission to build capacity and reduce structural inequalities. As such, the WHO supports low- and middle-income countries in developing their scientific, regulatory, and logistical capacities to enable them to effectively participate in the PABS system. It supports the transformation of national institutions into accredited PABS platforms and promotes the systematic inclusion of technology and know-how transfer clauses in SMTAs.
A mechanism for periodic and independent evaluation of the PABS system's performance has been established. The evaluation is conducted every two years, based on indicators relating to equity, transparency, speed of response, and contractual compliance. The results of this evaluation are made public.
Tunisia recommends that any substantial revision of the PABS rules, including the SMTA models, be subject to formal and inclusive consultation with the States Parties. The latter have a collective right to oppose any change that would compromise the fundamental principles of the system or the interests of the supplier countries.
VI. Benefit Sharing (Section B.6, Section G – Article 12.5(a), 12.8)
Expected benefits include:
Preferential access to health products (vaccines, tests, treatments) (details:
percentage, free of charge)
Technology transfer enabling local or regional production enabling more efficient production of medicines and vaccines, also considered as benefits, even if not purely financial
Funding for P3-level national laboratories;
Contractual transparency is ensured through a public database of manufacturers' commitments.
The Parties undertake to ensure a fair, equitable, and binding sharing of benefits arising from access to biological resources provided under the PABS system.
This sharing includes benefits:
Monetary (pecuniary): mandatory annual contributions to the PABS Fund,
proportional royalties in the event of commercialization;
Non-monetary (non-pecuniary): priority access to health products,
technology transfer, scientific co-development, and national capacity building.
Any manufacturer that has accessed pathogens must:
Commit to returning a portion of the products developed or profits to the country of origin;
Enter into an SMTA detailing these obligations;
Report annually on its deliveries, transfers, and contributions.
The WHO will then publish an annual report on compliance with sharing obligations, based on the SMTAs, manufacturer declarations, and feedback from State Parties.
Contractual transparency through a public database of manufacturer commitments is a powerful tool for improving commercial relations, building trust, and fostering a fairer and more competitive environment.
VII. Facilitation and acceleration of research and innovation:
Tunisia believes that the PABS system should not be limited to providing other countries with access to pathogens, but should also serve as a lever for scientific cooperation, skills transfer, and strengthening R&D sovereignty in middle-income countries.
Any access to biological resources or associated genetic data under the PABS system entails an obligation for the recipient to integrate, to the extent possible, the institutions of the provider country in research, product development, and scientific publication activities related to the analysis of the materials.
This obligation is formalized in the SMTAs and reflects the principles of co-development and co-publication.
A multilateral funding mechanism should be established within the PABS system, funded in particular by mandatory or voluntary contributions from beneficiary private entities. This fund supports collaborative research projects involving institutions in provider countries, based on competitive calls for proposals and criteria of scientific excellence, public health impact, and geographic relevance. Priority is given to research on endemic pathogens, the local production of diagnostic tools, and epidemiological surveillance systems.
Scientific data produced from resources obtained through the PABS system—including genetic sequences, experimental results, publications, potential patents, or derived innovations—must be shared with the WHO within a reasonable timeframe and made available, in an open, equitable, and non-discriminatory manner, for the benefit of public research institutions in the country of origin.
Since the SMTAs also include specific clauses relating to the transfer of technologies, know-how, and equipment essential for research, including reagents, cell lines, bioinformatics software, and validated protocols, these transfers will be accompanied, to the extent possible, by technical training and skills-building activities, at the beneficiary's expense, for researchers and technicians in the institutions of the provider country.
Also through the collaborative digital platform developed and administered by WHO as part of the PABS system, the networking of research teams from the Parties will enable the sharing of protocols, data, and publications from the system, and ensure transparent and accessible monitoring of research projects funded or derived from shared resources.
VIII. Complementarity between the PABS system and the WHO Pandemic Influenza Preparedness Framework (PIP) and other relevant ABS (Access and Benefit-Sharing) instruments,
The PABS system is designed to work in synergy with other relevant international access and benefit-sharing regimes, including:
The WHO Pandemic Influenza Preparedness Framework (PIP Framework);
The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits;
The International Treaty on Plant Genetic Resources (FAO);
Sequencing databases (GISAID, GenBank).
Nothing in this annex limits the sovereign rights of States under the Nagoya Protocol, without binding Member States that have not ratified this Protocol.
Tunisia strongly supports the idea that the SMTAs of the PABS system must be compatible with sharing agreements under other regimes. The WHO must ensure operational coordination, regulatory consistency, and the prevention of legal conflicts between instruments.
IX. Review and Alignment of National and Regional Access and Benefit-Sharing Measures
The Parties undertake to undertake a review of their national or regional legal frameworks relating to access to biological resources and benefit-sharing (ABS), to ensure their compatibility with this PABS system.
This review must be conducted in a sovereign, progressive manner, and in compliance with existing commitments, particularly those under the Nagoya Protocol.
The WHO, in collaboration with relevant partner organizations, will provide technical and legal support to assist States in this process, notably through guides, training, legal templates, and exchange platforms.
Tunisia, as a State Party, commits to coordinating efforts at the national and regional levels to strengthen the coherence of ABS measures within the framework of the international response to pandemics.
X. Consistency with Applicable Laws on Risk Assessment, Biosafety, Biosecurity, Export Control, and Data Protection
The provisions of the PABS system must be interpreted and applied in accordance with applicable national, regional, and international laws, including those relating to biosafety, biosecurity, biological risk assessment, export control of pathogenic material, and data protection, including sensitive genetic data.
Tunisia, in full respect of its sovereignty, will undertake to make access to pathogens or associated data subject to compliance with national frameworks on biosafety, privacy protection through the protection of personal data, and technology transfer regulations.
Standard Material Transfer Agreements (SMTAs) entered into under the PABS systemmust include a clause requiring the user to:
comply with the legal requirements of the supplier country regarding biosecurity, export control, and data confidentiality;
immediately notify the supplier of any security breach, traceability breach, or improper use;
not exploit for commercial purposes or transfer to a third party the data or materials received without the prior written consent of the country of origin.
XI. Facilitation of the Manufacturing and Export of Vaccines, Treatments, and Diagnostics
The Parties, including Tunisia, undertake to facilitate rapid, equitable, affordable, and public health-needs-based access to vaccines, treatments, and diagnostics developed from biological materials or data obtained through the PABS system.
Any entity benefiting from the PABS system, whether public or private, is required to include in the SMTA a clause stipulating:
the provision of a specified share of final products (vaccines, treatments, diagnostics) to supplier countries, free of charge or at an affordable cost, particularly in the event of a public health emergency;
the prioritization of low- and middle-income countries, particularly those in the Global South, in distribution strategies during pandemic situations.
Beneficiaries are encouraged, and in some cases required, to enter into local or regional manufacturing partnerships, including activities such as packaging, filling and finishing, distribution, and quality assurance.
They also commit to sharing production protocols, critical tools, validated cell lines, and quality validation processes. In exceptional circumstances, the Parties agree to temporarily lift certain legal barriers to access to essential technologies, including through the use of compulsory licenses, voluntary access mechanisms, or other flexibilities provided for in the TRIPS Agreement, in accordance with WTO decisions.
Tunisia maintains that any country that has contributed to the PABS system by sharing biological resources or genetic data has the right to:
receive a guaranteed minimum quota of products developed from these resources (in kind or otherwise);
be included in manufacturing partnerships, based on its national or regional industrial capabilities;
request technical or financial support to upgrade its pharmaceutical production capacities.
Section C: Traceability and Open Access to Data (Article 12.3)
Traceability and open access to data are technical but highly strategic elements of the PABS system: they determine its transparency and effectiveness.
A centralized digital traceability system, managed by the WHO, enables real-time tracking of the movements of pathogen resources or their sequences within the PABS system.
All SMTAs concluded within this framework must be registered with the WHO in a secure registry, with a publicly accessible non-confidential summary.
Beneficiary users are required to declare:
The use made of resources,
The research results obtained,
The health products developed,
Any patent or license applications.
The scientific data resulting from this research must be shared on open, interoperable platforms accessible to provider countries, unless otherwise justified.
Section D: Alignment with the Nagoya Protocol
Tunisia, as a Party to the Nagoya Protocol, which it ratified in 2021, commits to ensuring consistency between the provisions of the PABS system and its international obligations relating to access to genetic resources and the fair and equitable sharing of benefits arising therefrom.
The State Party shall be supported through the designated national focal point for the Nagoya Protocol in developing these measures and aligning them with relevant multilateral frameworks, including this instrument and its annex.
Section E: Provisions in the Event of a Declaration of a Pandemic Emergency (Art. 12.6)
Providing countries must benefit from rapid, effective, equitable, and undelayed access to health products (vaccines, treatments, diagnostics).
In the event of a pandemic emergency declaration by the World Health Organization, a mechanism for accelerated and coordinated access to health products derived from the PABS system is automatically activated, without delay.
The products concerned include, but are not limited to:
vaccines,
therapeutic treatments,
diagnostic and rapid detection means,
as well as protection and response tools developed from biological resources shared through the PABS system.
The distribution of these products is coordinated by the WHO and its partners, based on:
a real-time scientific assessment of epidemiological risks,
the urgent health and humanitarian needs of populations,
national response capacities.
Particular attention is paid to least developed countries (LDCs), small island developing states (SIDS), and low-income countries to ensure priority, inclusive, and equitable access.
Countries that have provided a pathogen or its genetic data that caused the pandemic emergency benefit from a priority quota of health products, equivalent to at least 10% of the available global production of each type of derived product, delivered within 30 days of their operational availability.
Tunisia, as a State Party, undertakes to:
not apply export restrictions or protectionist measures to these products throughout the duration of the pandemic,
facilitate the issuance of compulsory or voluntary licenses, if necessary, to accelerate production and distribution worldwide.
The WHO, with partner international agencies, coordinates logistical, financial, and technical aspects to ensure effective, equitable, and free or low-cost delivery, particularly to vulnerable countries.
Section F: Provisions Applicable in the Event of a Public Health Emergency of International Concern (PHEIC):
In the event of a declaration by the WHO of a Public Health Emergency of International Concern (PHEIC), the following PABS obligations are automatically activated and without preconditions:
Immediate and effective access by Parties to health products developed from resources shared through PABS (vaccines, treatments, diagnostics);
Prioritization based on an assessment of public health risks and proven health needs, with particular attention to least developed countries, island states, and pathogen-supplying countries;
The obligation for SMTA beneficiaries to implement expedited delivery and effective sharing of products in accordance with contractual commitments;
Temporary exemption from any export restriction or health protectionism that undermines equity of access.
The cost of products allocated under this framework must be covered by the PABS Multilateral Fund, or offered at an affordable, non-profit price, determined in consultation with the WHO.
The WHO and its partners coordinate logistics, including transportation, distribution, and technical support for recipient countries.
A public database is maintained by the WHO, listing:
the countries that received the products,
the quantities delivered,
and the contractual commitments fulfilled.
In the event of non-compliance with commitments during a PHEIC, several measures may be considered, including: suspension of access to the PABS system, publicity of the breaches observed, and recourse to the dispute settlement mechanism provided for by the instrument.
Section G: Additional Provisions on Benefit-Sharing (Art. 12.8)
These are additional provisions, to be included in the PABS Annex, to ensure that benefit-sharing is not merely symbolic or minimal, but supports a sustainable transformation of national capacities.
Tunisia plans to implement strengthened, flexible, yet binding benefit-sharing mechanisms, particularly in:
training,
technology transfer,
regional co-production,
intellectual property ownership,
sharing of preclinical and clinical knowledge.
The Parties undertake to include in the SMTAs a clause for the progressive transfer of technology, particularly for the production, formulation, or packaging of vaccines, diagnostics, or treatments, to supplier countries or their regional partners.
Recipients of PABS resources must share protocols, results, and analyses of preclinical and clinical trials with interested parties, within a timeframe consistent with the interests of public health.
When technically and legally justified, the institutions that provided the biological material or associated data may be recognized as co-owners of the intellectual property or granted non-exclusive access, under a free or preferential license.
Any scientific results published using resources from the PABS system must explicitly acknowledge the source of the resource and, where possible, include institutions or researchers from the providing countries as co-authors or collaborators.
A percentage of the mandatory monetary contributions paid by users will be allocated to a window intended to finance research, development, or manufacturing projects in the providing countries. Any commercial exploitation of a product derived from PABS resources must be subject to prior notification to the supplier country and include a negotiated sharing of profits or royalties, in accordance with the SMTA.
Section H: Other elements for effective operationalization of the PABS system (Art. 12.9)
For the PABS system to be fully functional and not remain an abstract framework, the annex should include clear, concrete, and measurable operational elements that guarantee:
its rapid implementation upon entry into force of the treaty,
its technical and logistical efficiency,
its accessibility for all countries, including Tunisia.
The Parties recognize that the effectiveness of the PABS system depends on the rapid, equitable, and coordinated implementation of its institutional, technical, digital, and logistical components. Accordingly, the Parties agree to establish the following mechanisms within a specified timeframe, commencing upon the entry into force of this instrument.
The World Health Organization shall establish a permanent PABS coordination unit, responsible for:
the centralized management of Standard Material Transfer Agreements (SMTAs);
monitoring the circulation of pathogens and their associated data;
overseeing the PABS digital platform;
coordinating with the Parties and relevant stakeholders.
A secure digital platform is established and administered by the WHO to:
facilitate the registration, signing, and monitoring of SMTAs;
ensure the traceability of PABS resources and derived products;
ensure transparency of user commitments and accountability;
enable public access to non-sensitive scientific data, in compliance with national data protection and biosafety legislation.
Parties may designate, in consultation with the WHO, accredited national and/or regional laboratories to ensure the collection, storage, sequencing, and, where appropriate, transfer of pathogens under the PABS system. These laboratories must meet harmonized biosafety, biosecurity, and quality standards.
A harmonized and legally binding SMTA model is being developed by the WHO, in consultation with the Parties. This model must include:
modalities for pathogen transfer and use;
modalities for the fair and equitable sharing of pecuniary and non-pecuniary benefits;
specific obligations applicable in pandemic emergencies;
clauses on traceability, prohibition of unauthorized re-export, and non-reverse engineering;
conditions for notification, transparency, and contractual liability.
An international capacity-building program is being implemented under the aegis of the WHO to support States Parties with limited capacity, particularly in:
biosafety and biosecurity;
legal and technical management of SMTAs;
use of the digital platform;
mastery of tools for sequencing, storage, and analysis of pathogens.
The WHO shall ensure the establishment of an annual or periodic monitoring and evaluation system, including public and verifiable indicators relating to:
the number and nature of shared PABS resources;
SMTAs signed and implemented;
the amounts and modalities of benefit sharing;
the effective distribution of medical countermeasures during public health emergencies.
The Parties agree to conduct, every two (2) years, a technical evaluation and review of the PABS system, conducted by an independent body appointed by the Conference of the Parties. This review shall allow for the adaptation of the system's operational tools without modifying the substantive provisions of this instrument.


























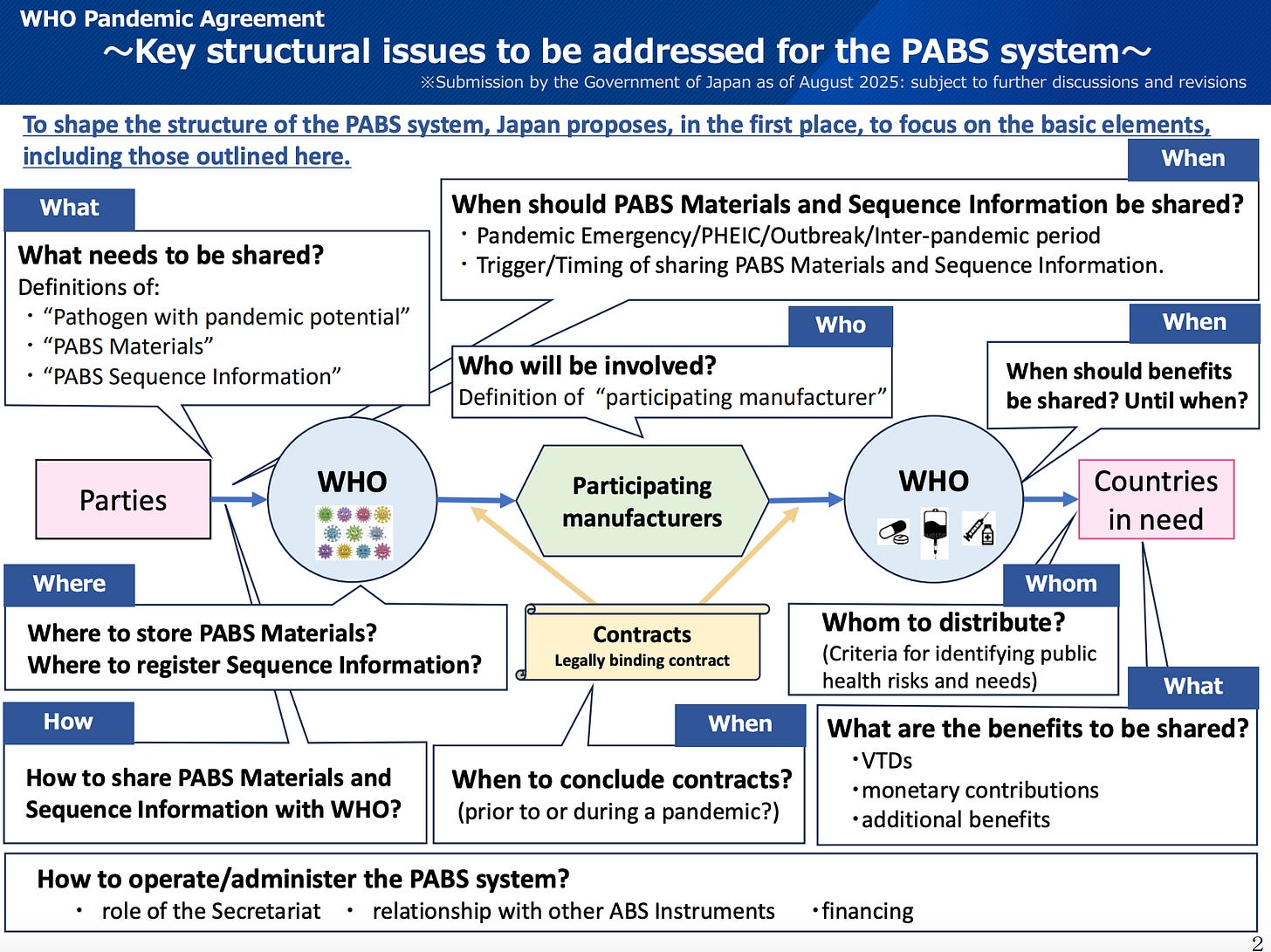









Firstly, I would like to thank James for his efforts in repeatedly exposing and thoroughly investigating these mega-crimes against humanity. The gruesomely “funny” thing about this medical aspect of the global power grab by the NWO scum is that they base everything on an assumption. The “fact” that pandemics exist. At most, there have been epidemics, but not pandemics. So that alone should be reason enough to shut down the entire WHO and arrest all its employees and financiers and lock them up for life.
Thanks for your attention to detail James!