ModeRNA
Is ModeRNA planning to commit fraud? Tune in this Tuesday (June 14, 2022) and Wednesday (June 15, 2022) to watch the VRBPAC meetings to see for yourself.
SORRY MODERNA, YOUR REQUEST MUST BE DENIED!
CLICK HERE TO LEARN ABOUT THE PFIZER PFRAUD!
“We the People” do not appreciate your attempt to deceive us. Your misrepresentation of the data from your clinical trials is clearly fraudulent.
Below is the letter that the FDA MUST, by law, send to ModeRNA to reject their request for Emergency Use Authorization of their injectable “biological product.”
Dear ModeRNA,
In order for the FDA to authorize any biological product for emergency use, THE LAW REQUIRES THAT ALL OF THE FOLLOWING CRITERIA BE PROVEN:
EMERGENCY: There must be an emergency involving a disease that poses a risk of death to the target patient demographic.
EFFECTIVENESS: The biological product being considered must be shown to be effective in PREVENTING the deadly disease.
SAFETY: The biological product being considered must be shown to be safe.
POSITIVE BENEFIT/RISK RATIO: The benefits of the biological product must outweigh the risks.
The evidence below shows that ModeRNA has FAILED to prove all of the above mandatory criteria, so the FDA has no other legal recourse than to reject ModeRNA’s request for Emergency Use Authorization of their biological product.
Please watch the videos below…
Backup video:
https://rumble.com/v189ihy-fauci-ouchie-for-infants.html
https://rumble.com/v18daq7-then-they-came-for-our-babies.html
It is time to wake up:
It is time to speak up:
My request to all of my readers:
Watch the videos above.
Inform yourself by reading this article.
Pray (literally) for EVERYONE to awaken to the truth.
CLICK HERE to review the meetings’ agenda.
Watch the VRBPAC meetings (live or recorded).
CLICK HERE to send a comment to your Senators, Congressional Representative, Governor and State Assembly Representative.
CLICK HERE to read Robert F. Kennedy’s statement.
CLICK HERE to for the official FDA announcement.
Contact me if you have any questions (310-619-3055).
Share this article with everyone you can A.S.A.P.
NOTE: This article primarily analyzes the information regarding ModeRNA’s data about children ages 6 months to 5 years.
SAVE THE DATES: June 14-15, 2022
REVISIT THIS PAGE TO WATCH BOTH OF THE VACCINE ADVISORY COMMITTEE MEETINGS LIVE STREAMED OR RECORDED:
Tuesday June 14, 2022 5:30 am Pacific Time
VOTING QUESTIONS FOR MODERNA
Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine when administered as a 2-dose series (100 μg each dose) outweigh its risks for use in adolescents 12 through 17 years of age?
Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine when administered as a 2-dose series (50 μg each dose) outweigh its risks for use in children 6 through 11 years of age?
Wednesday June 15, 2022 5:30 am Pacific Time
VOTING QUESTION FOR MODERNA
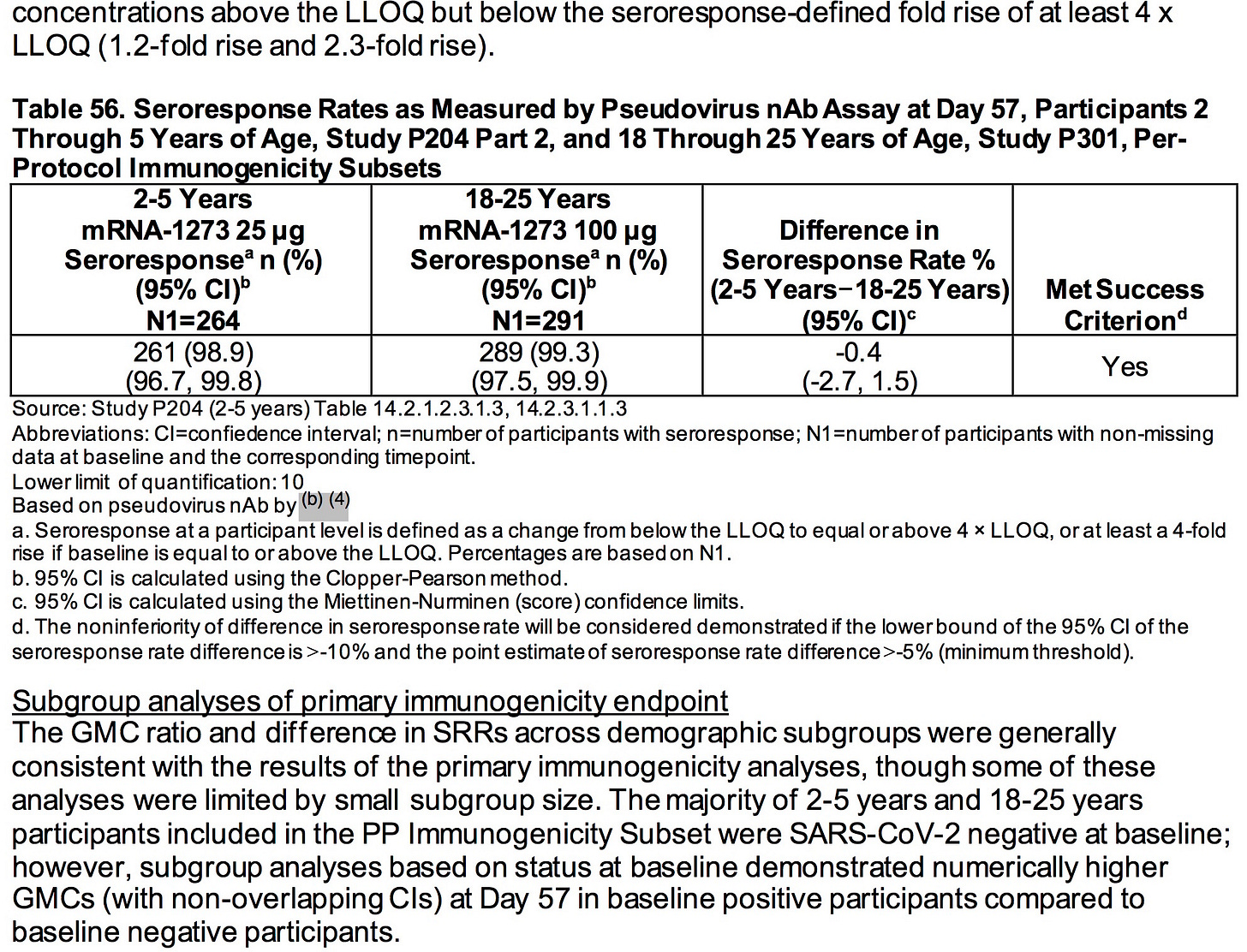
Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine when administered as a 2-dose series (25 μg each dose) outweigh its risks for use in infants and children 6 months through 5 years of age?
VOTING QUESTION FOR PFIZER-BioNTech
Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine when administered as a 2-dose series (3 μg each dose) outweigh its risks for use in infants and children 6 months through 4 years of age?
Watch the meetings and see if the members of the Vaccine Advisory Committee raise any of the issues below regarding ModeRNA’s biological product for children 6 months to 5 years of age.
ModeRNA FAILED to structure their clinical trial to determine whether or not their biological product would reduce death due to COVID-19.
ModeRNA FAILED to structure their clinical trial to determine whether or not their biological product would reduce severe COVID-19.
ModeRNA has FAILED to provide a calculation for the number of children needed to inject in order to save even one life, because their clinical trial FAILED to show that their biological product saves lives.
ModeRNA has FAILED to explain why 38.5% (678/1761) of the children (6 to 23 months) who received their biological product suffered an adverse event that required medical attention.
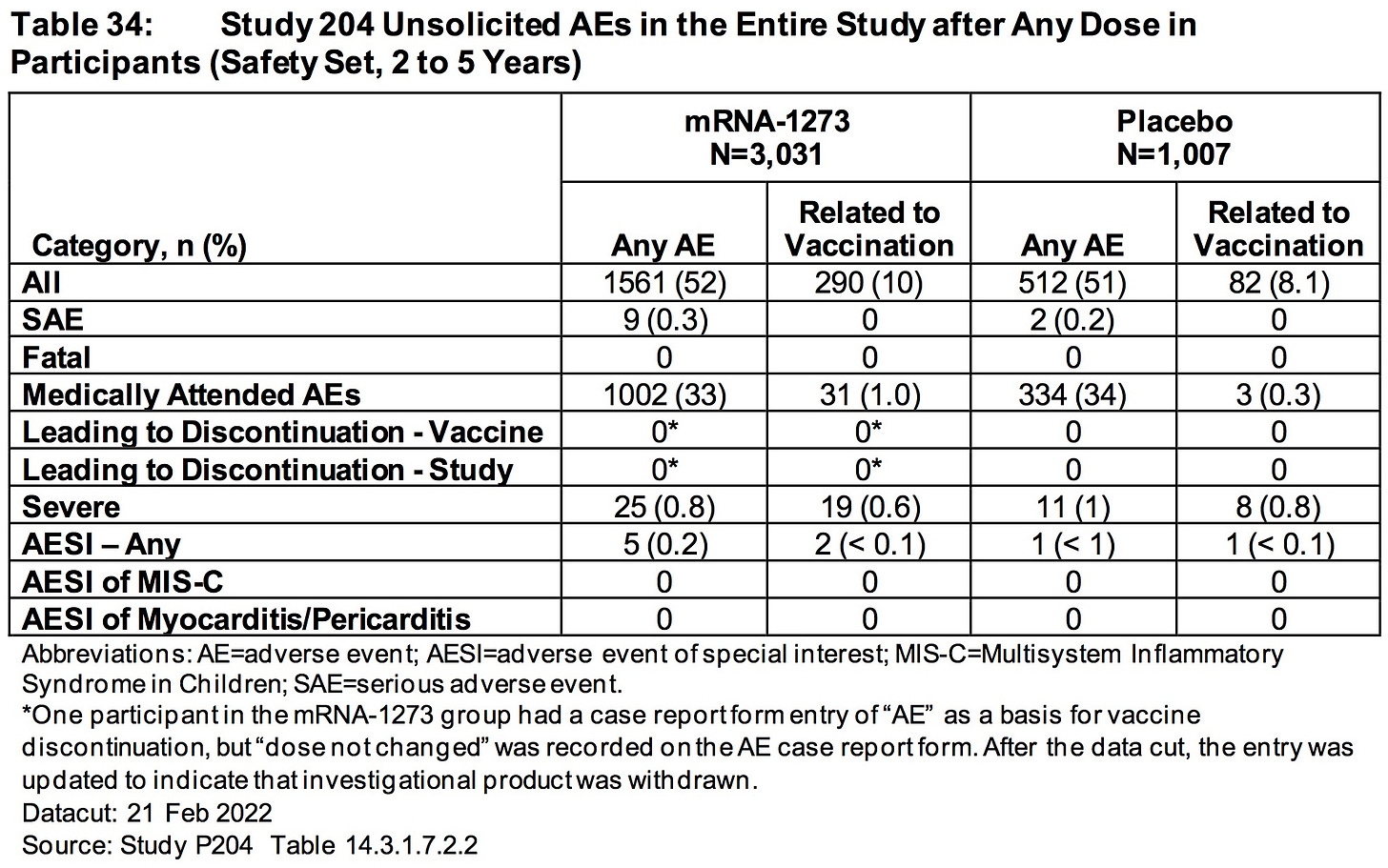
ModeRNA has FAILED to explain why 33% (1002/3031) of the children (2 to 5 years) who received their biological product suffered an adverse event that required medical attention.
ModeRNA has FAILED to explain why the same dose (25 mcg) was given to children in the 6 to 23 month age group as was given to the 2 year to 5 year age group. Moderna has decided to give the same sized dose to a 6 month old infant (average weight: 17 pounds) as a 5 year old child (average weight: 40 pounds).
ModeRNA has FAILED to explain why the children (6 to 23 months) who received their biological product were 294% as likely to suffer SEVERE ADVERSE REACTIONS (.85%) as children in the 2 to 5 year group (.29%). Think about it. Is it any surprise that the overdosing of infants resulted in greater numbers of SEVERE ADVERSE REACTIONS?
ModeRNA has FAILED to explain why the children (6 to 23 months old) who received their biological product were 500% as likely to suffer SEVERE ADVERSE REACTIONS as those who received placebo (.85% versus .17%) .
ModeRNA has FAILED to explain why the children (2 to 5 years old) who received their biological product were 342% as likely to suffer SEVERE ADVERSE REACTIONS attributed to Moderna’s biological product as those who received placebo (1.02% versus .298%) .
ModeRNA has FAILED to explain why 433% as many participants (6 to 23 months) who were injected with their biological product contracted croup when compared to those who received placebo.
ModeRNA has FAILED to explain why over 400% as many participants (2 to 5 years) who were injected with their biological product contracted Respiratory Syncytial Virus (RSV) when compared to those who received placebo.
ModeRNA has FAILED to show that its biological product prevents infection.
ModeRNA has FAILED to show that its biological product prevents disease, as required by law.
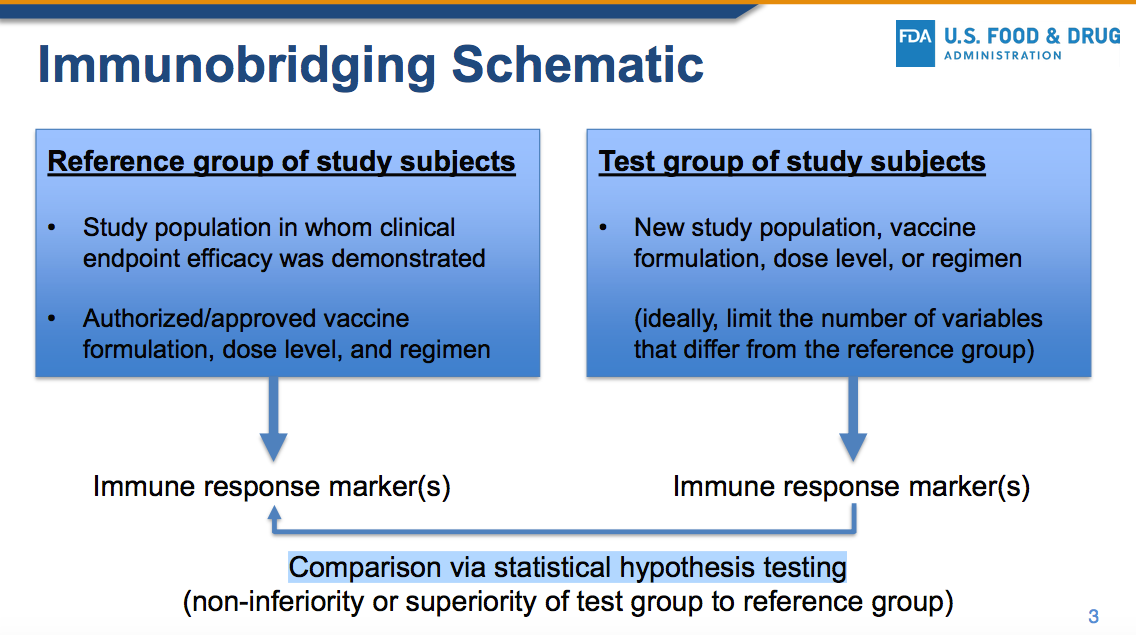
ModeRNA focused instead on the questionable concept of “immunobridging” which has NOT been codified into the law, nor into the regulations.
ModeRNA focused instead on “cases” that were primarily determined by RT-PCR testing, and they did NOT specify the number of cycles used.
ModeRNA’s claim of “effectiveness” in reducing COVID-19 “cases” ranged from 31-51%. Any of the “cases” of COVID-19 in young children obviously resolved themselves in a manner in which the placebo recipient survived and developed robust, long term immunity.
ModeRNA has FAILED to prove that the vaccine is safer or more effective at preventing serious COVID-19 or any COVID-19 in children COMPARED TO THE ALTERNATIVES. We are all familiar with the use of Vitamin D, HCQ and IVM as effective prophylaxis. The vaccine has not been proven to be safer or more effective that these (or other) alternatives. [Submitted by Mark Brody]
ModeRNA has FAILED to provide evidence of safety and efficacy of any type DONE BY INDEPENDENT RESEARCHERS. ModeRNA data can not be admitted as legitimate evidence as they are inextricably biased towards their own data. They have no INDEPENDENTLY CONDUCTED RESEARCH demonstrating the safety and efficacy of their products. [Submitted by Mark Brody]
Below is ModeRNA’s fairy tale “Benefits and Risks Conclusions” which has been extracted from their 118 page document.
I am so absolutely disgusted by the obvious attempt by ModeRNA to deceive the VRBPAC, the FDA and the general public with their absolutely ridiculous and fraudulent risk/benefit analysis that I wrote my own version in order to help to expose their pharmaceutical fraud and data manipulation via “modeling” to the entire world.
MODERNA DOCUMENTS:
https://www.fda.gov/media/159157/download
https://www.fda.gov/media/159189/download
https://clinicaltrials.gov/ct2/show/NCT04796896
THE LAW:
https://www.law.cornell.edu/uscode/text/21/360bbb-3
One of the ways that Moderna and the FDA rig the game is by adding endless layers of complexity to hide how bad the data really is. This should have been four separate documents — Moderna in adolescents 12 to 17, Moderna in kids 6 to 11, Moderna in kids 2 to 5, and Moderna in kids 6 months to 23 months. Looked at individually, the shot fails in each of these four age groups. But by lumping them together it creates noise that makes it difficult to understand what’s going on.
Another really pernicious thing that Moderna does is to further subdivide these populations into eight different subpopulations (Randomization Set, Full Analysis Set, Immunogenicity Subset, Per-protocol Immunogenicity Subset, Per-protocol Set for Efficacy, Modified Intent-to-treat Set, MITT1 Set, Safety Set, Solicited Safety Set).
See what they did there? The public just wants to know — does the product work and what are the side effects? By dividing the data into eight subcategories involving four different age groups now you have to wade through 32 different tables to try to make sense of what happened in the clinical trial.
They do something similar with the adverse events by dividing it across five tables x four age groups = 20 adverse event tables in all.
Subdividing the data in this way also allows Moderna to eliminate or hide data that it does not like. This is what people call “massaging the data” and it is unethical and a violation of scientific norms.
SOURCE:
1. THERE IS NO EMERGENCY
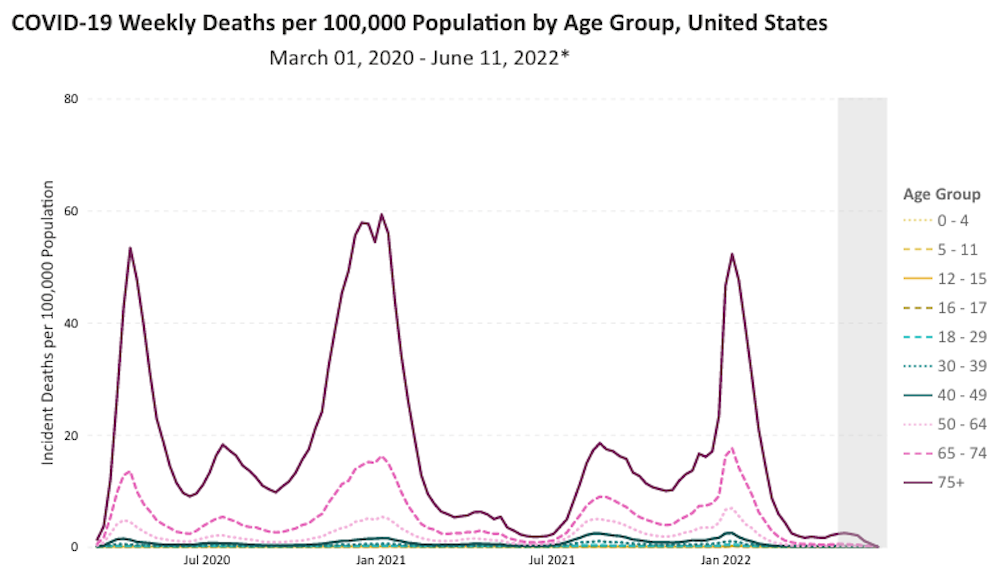
Children simply do not die from COVID-19.
https://covid.cdc.gov/covid-data-tracker/#demographicsovertime
The lowest risk was observed in children aged 5-11 without comorbidities. In this group, the ICU admission rate was 0.2 per 10,000 and case fatality could not be calculated, due to an absence of cases.
SOURCE:
https://www.medrxiv.org/content/10.1101/2021.11.30.21267048v1
Deaths among children under the age of 6 years have actually decreased.
There is no evidence of state of emergency in regard to COVID-19 in children that are older than 6 months and younger than 6 years.
In 2020, increased under-5 deaths were anticipated from the repercussions of strained and under-resourced health systems, limitations on care-seeking and preventative measures like vaccination and nutrition supplements, or socioeconomic strains on households resulting from job losses, economic contractions or even deaths of parents due to COVID-19.
Analysis data from 80 countries, coming from civil registration and vital statistic systems (CRVS), health management information systems (HMIS) as well as specific country-wide monitoring systems (Mozambique and South Africa) indicate no significant deviation from expected mortality for 2020 and in some cases reported fewer deaths than would be expected from historical data.
SOURCE:
Moderna is in a race against natural immunity. But natural immunity has already won because 74.2% of kids had natural immunity by February — so by now the number is probably closer to 100%. The God-given immune system in kids has already done its part to stop the pandemic and now the FDA wants to mess that up to enrich the cartel and keep the pandemic going forever.
68% of children aged 1-4 years old have apparently already been exposed to SARS-CoV-2 and have developed natural immunity.
Pediatric SARS-CoV-2 seroprevalence increased substantially over the study period, reaching 68% (95% CI: 63-72%) among children aged 1-4 years
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4092074
2. MODERNA’S BIOLOGICAL PRODUCT HAS NOT BEEN PROVEN TO BE EFFECTIVE IN PREVENTING SEVERE COVID-19 OR DEATH.
Effectiveness must be defined as it is in the law in regards to PREVENTING disease, not merely mitigating the symptoms or altering some blood-borne biological marker when compared to a study in a different group (see immunobridging below).
(2) that, based on the totality of scientific evidence available to the Secretary, including data from adequate and well-controlled clinical trials, if available, it is reasonable to believe that—
(A) the product may be effective in diagnosing, treating, or preventing—
(i) such disease or condition; or
SOURCE:
https://www.law.cornell.edu/uscode/text/21/360bbb-3
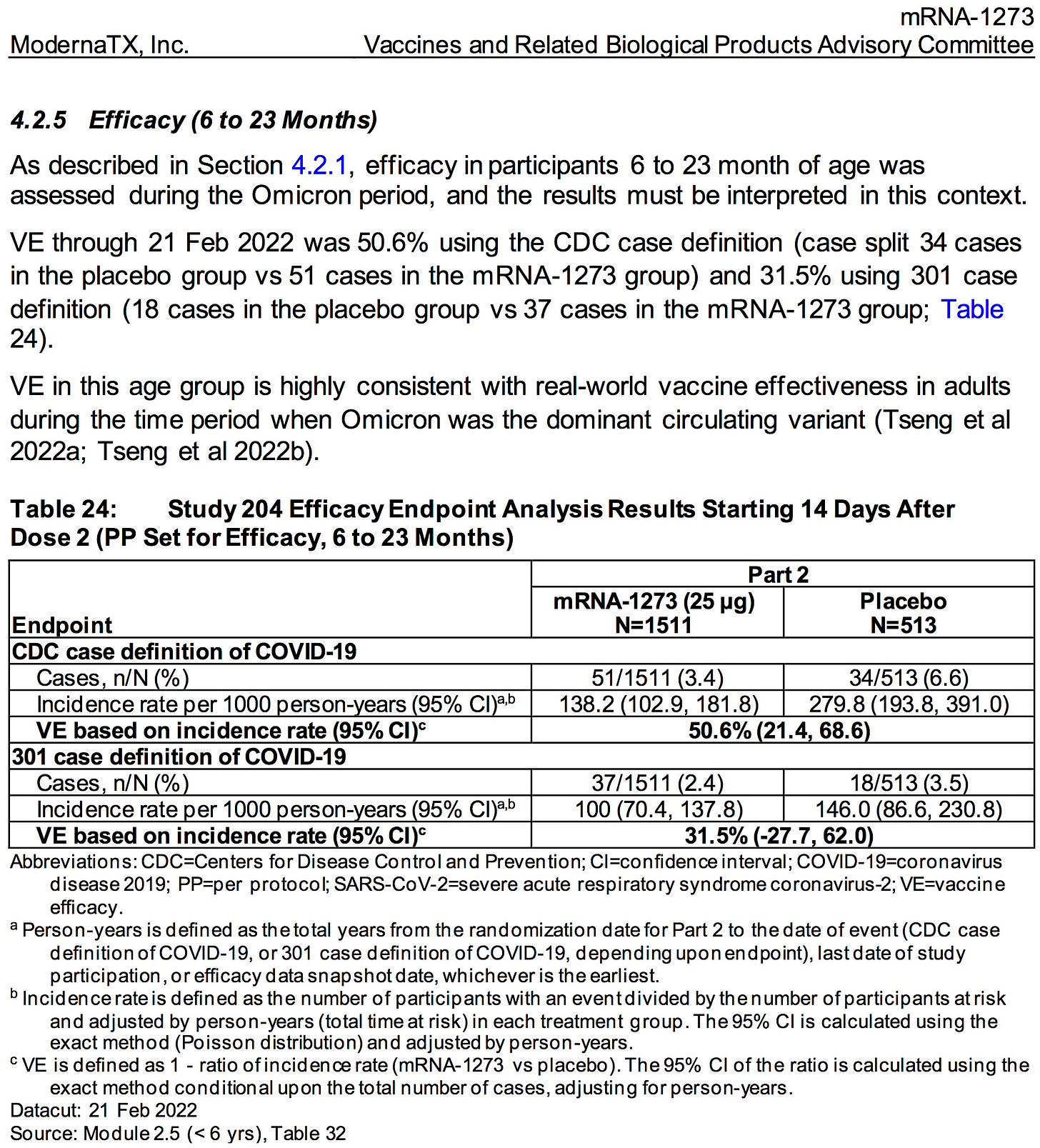
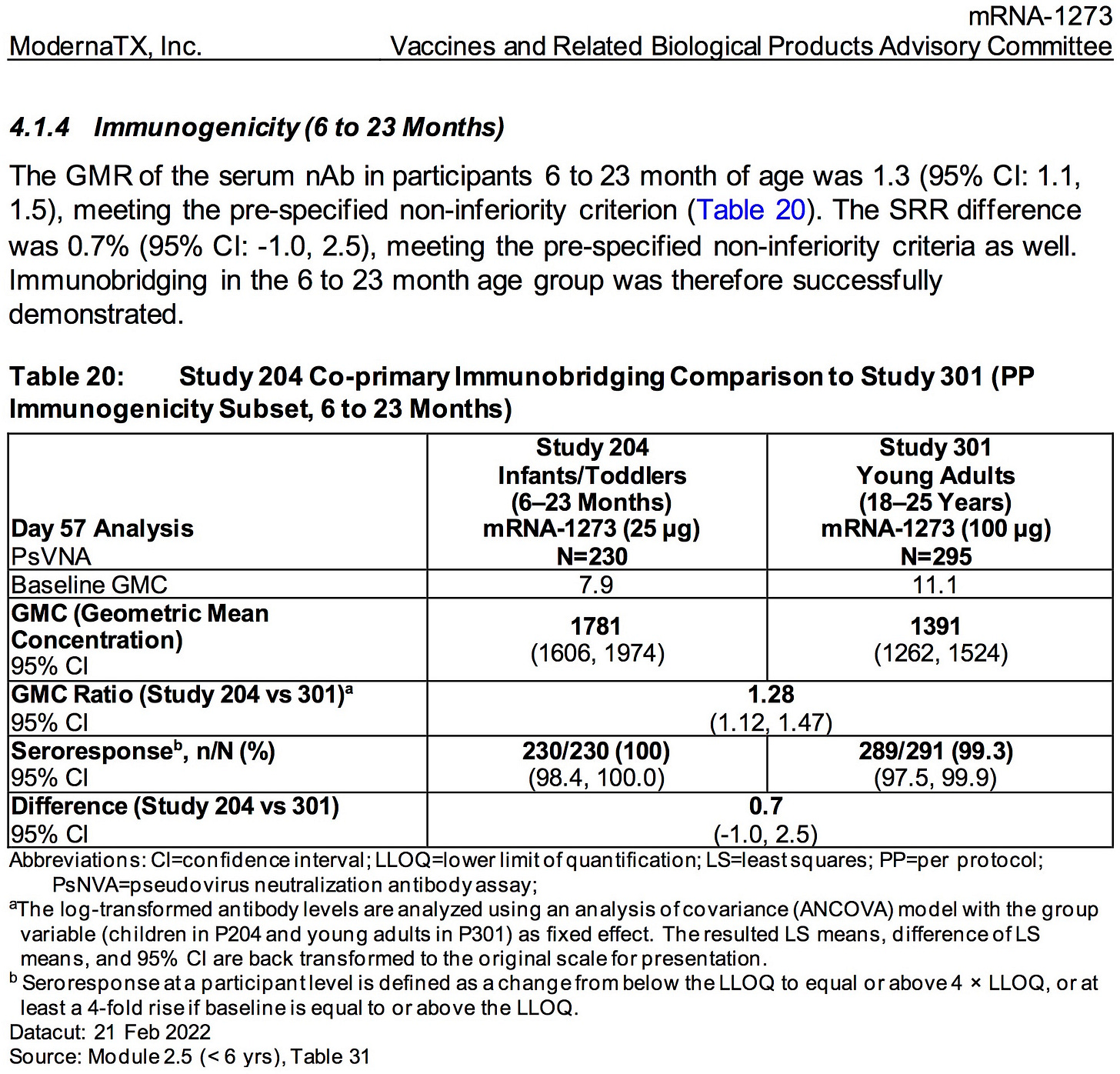
AGES 6 MONTHS TO 2 YEARS
SOURCE:
https://www.fda.gov/media/159157/download (pages 53)
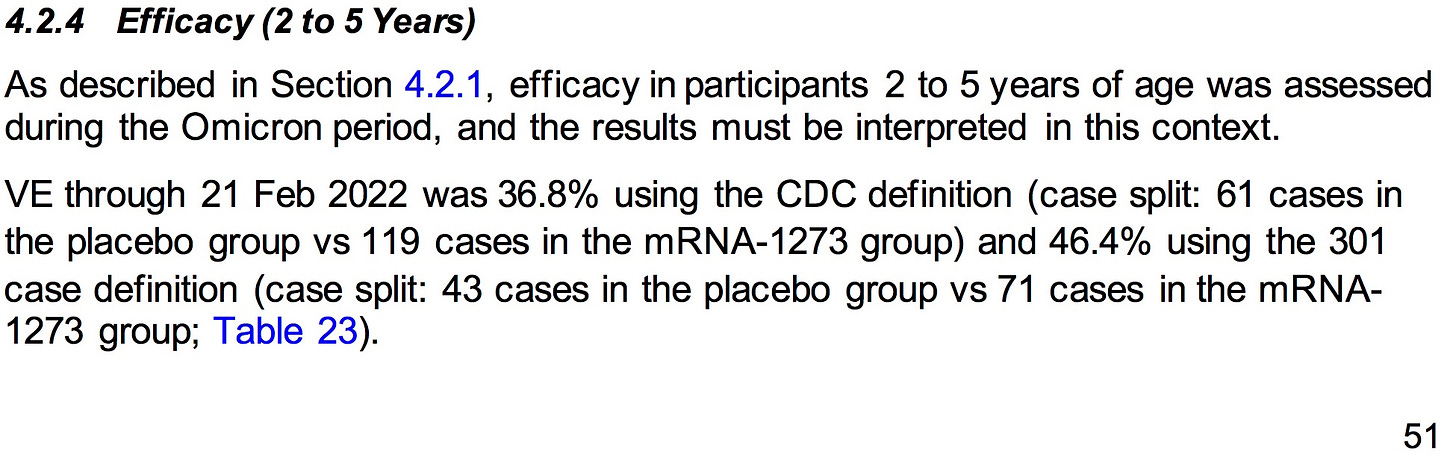
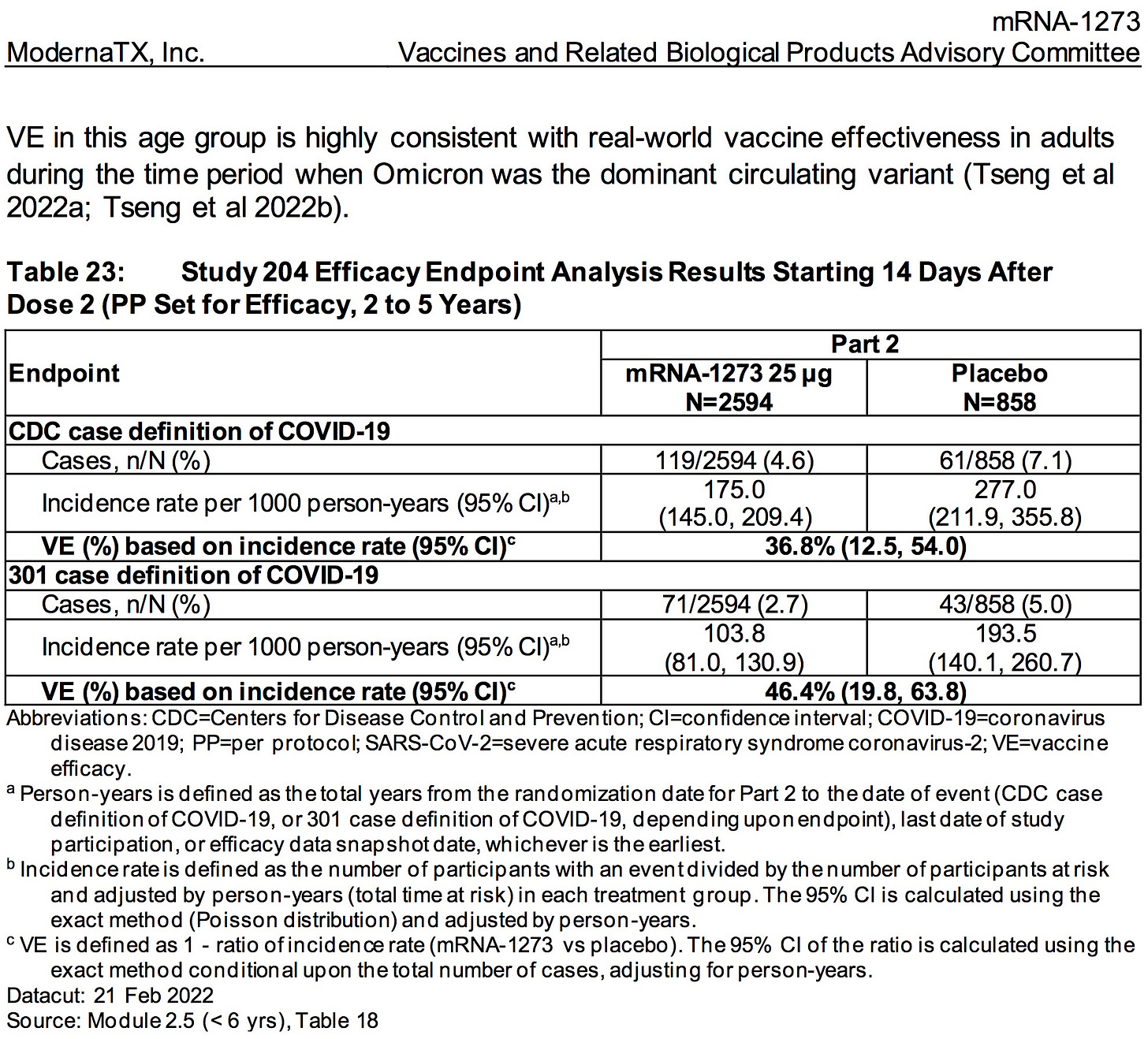
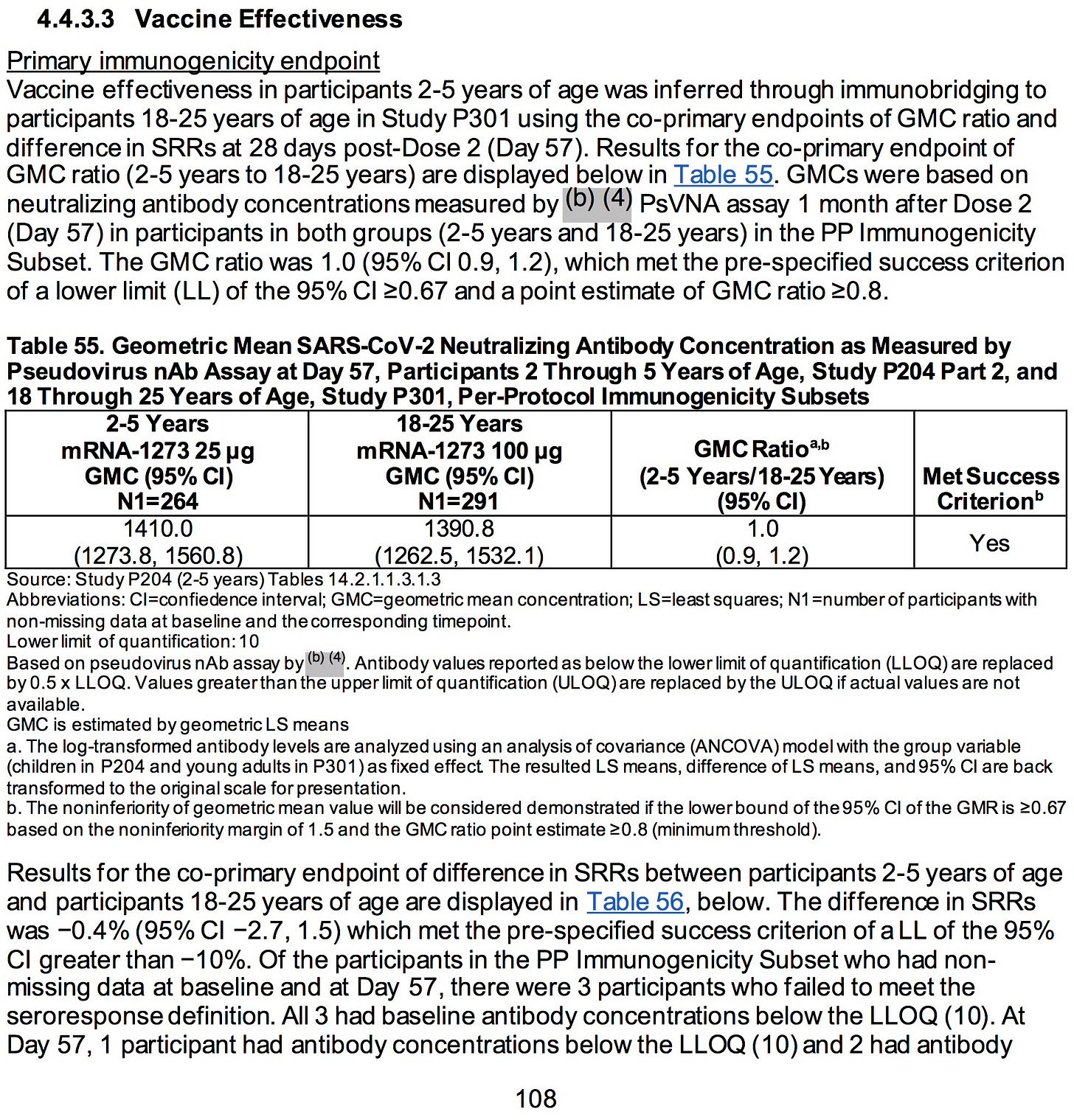
AGES 2-5 YEARS:
SOURCE:
https://www.fda.gov/media/159157/download (pages 51-52)
3. TO CLAIM THAT THE MODERNA BIOLOGICAL PRODUCT IS SAFE IS ABSOLUTELY ABSURD.
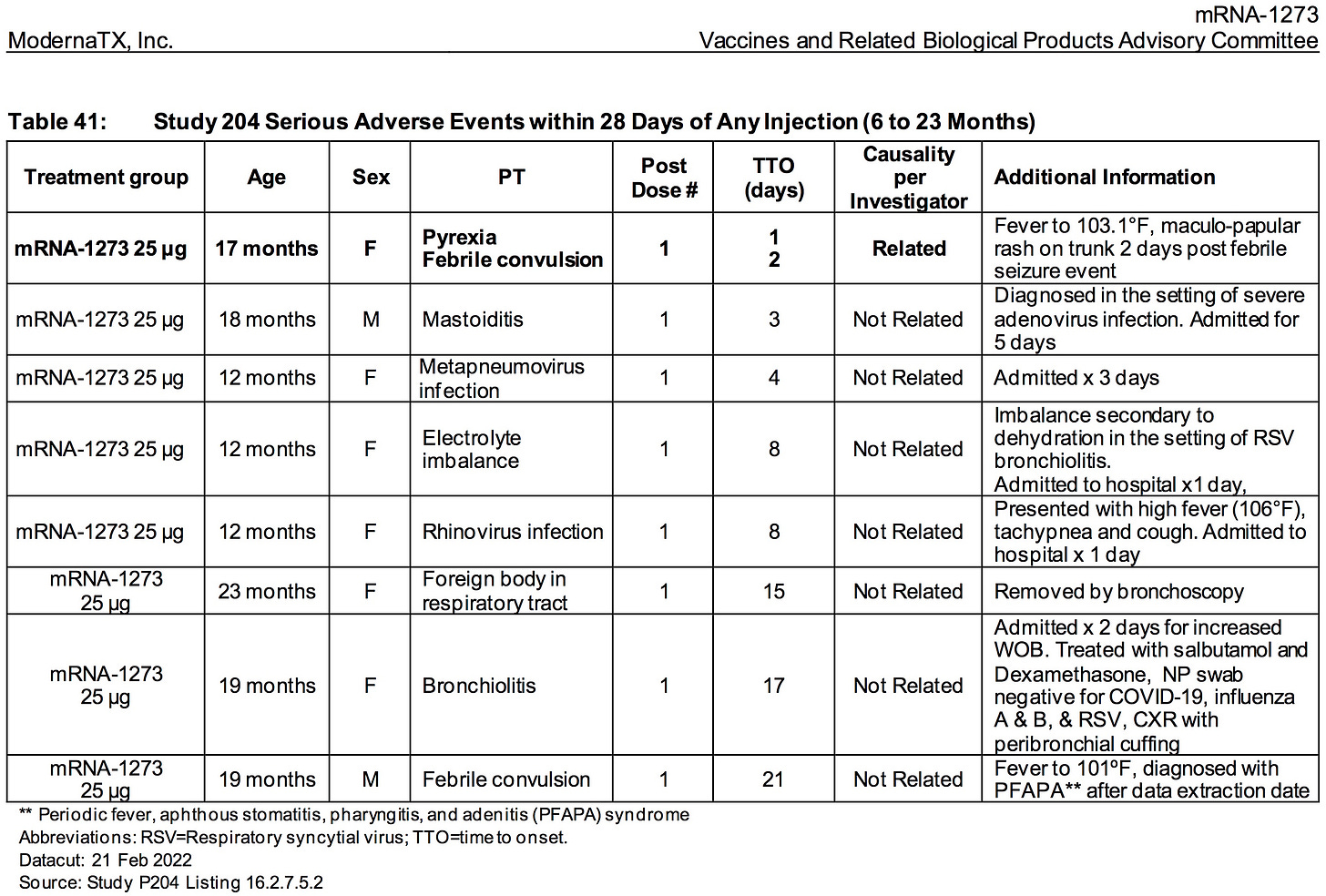
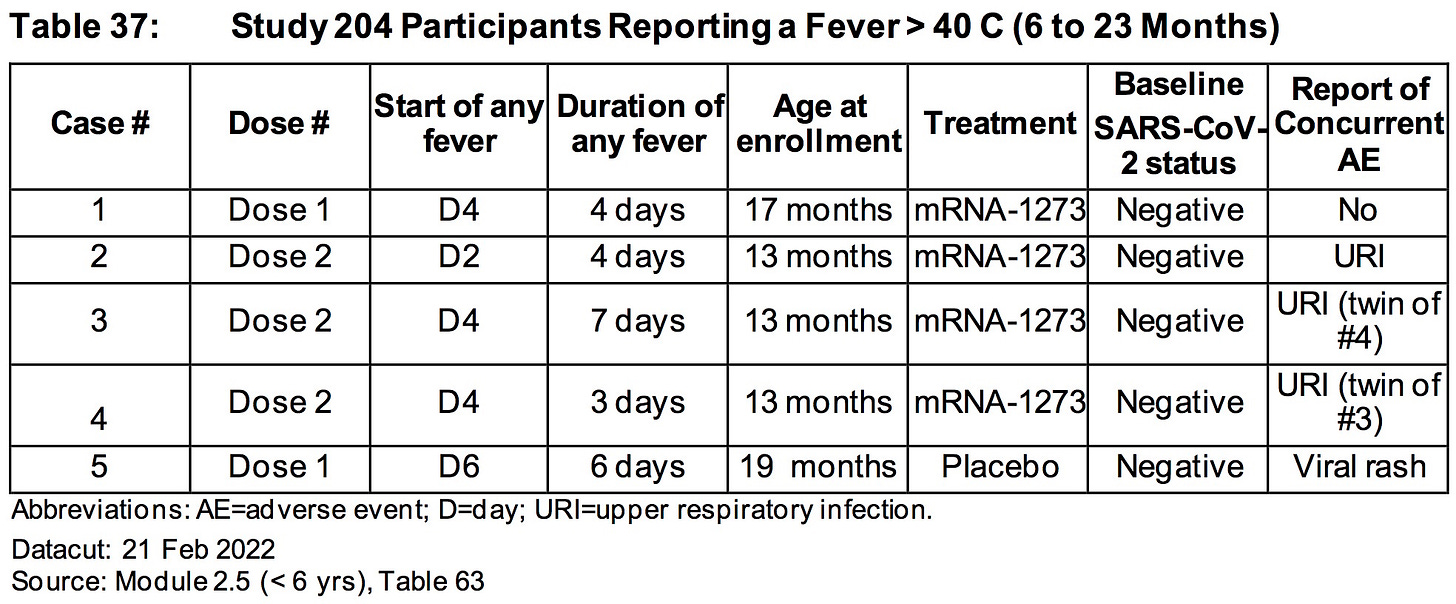
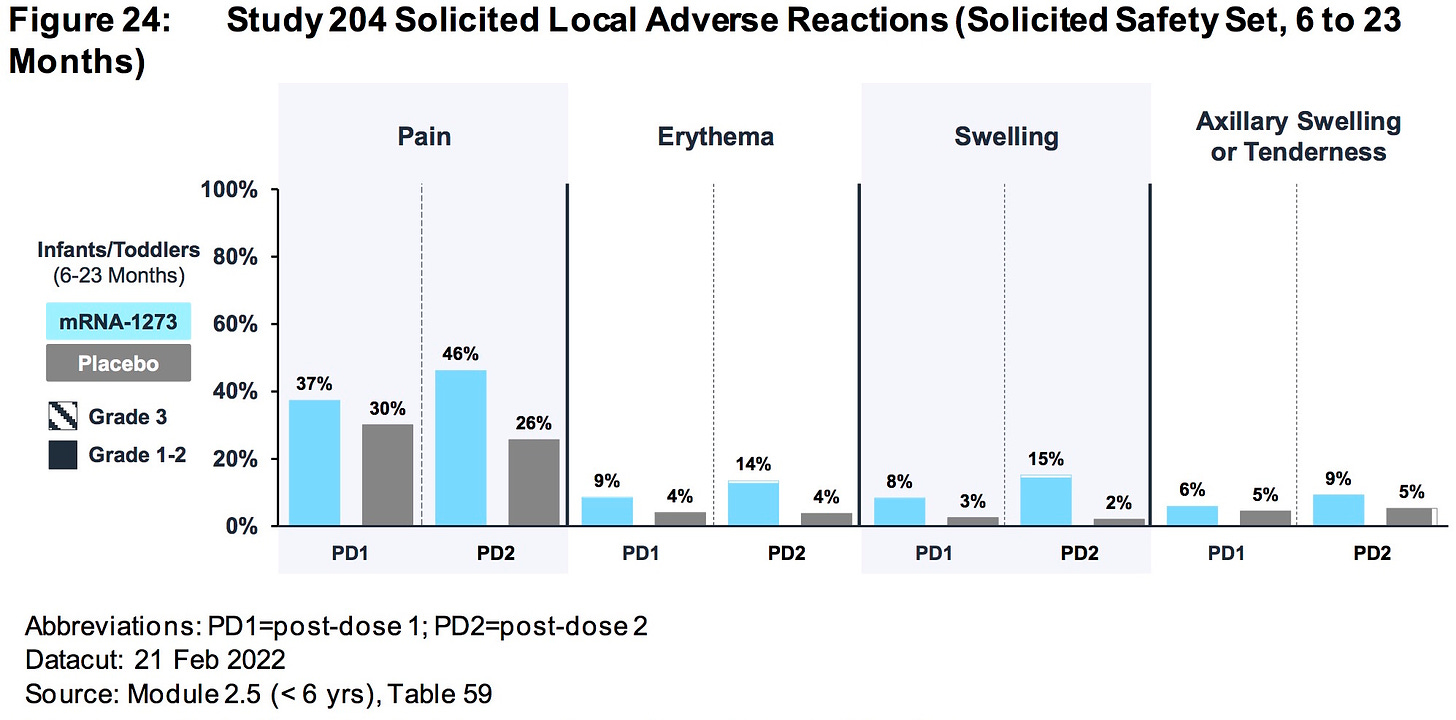
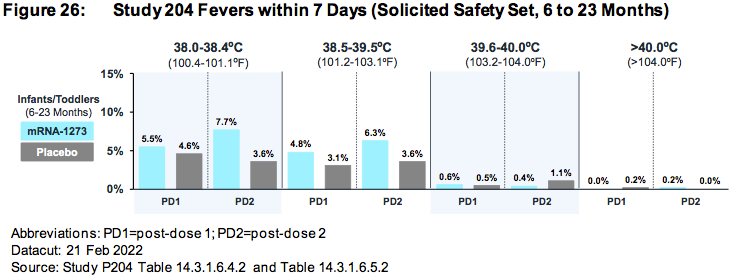
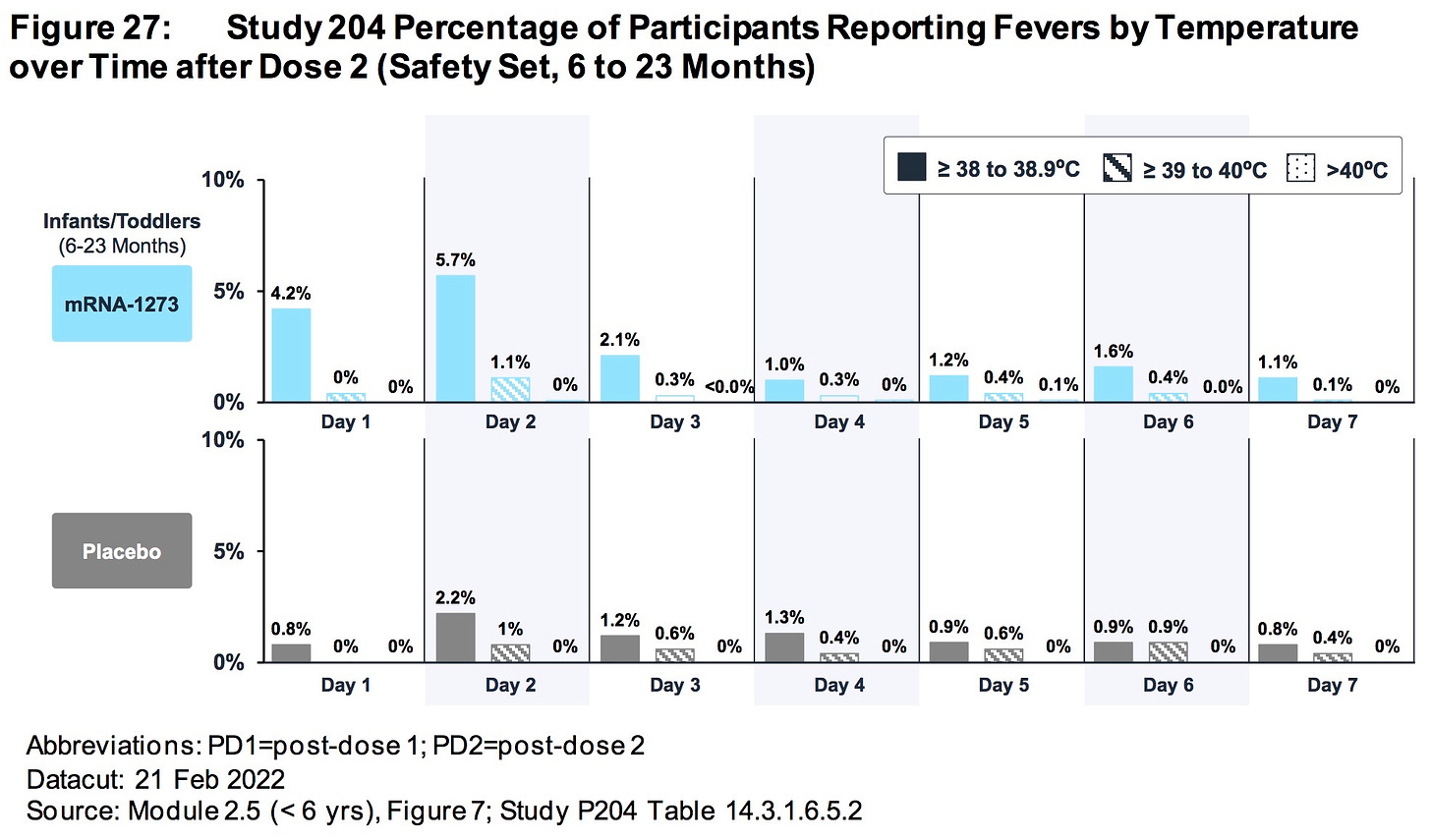
AGES 6 TO 23 MONTHS:
The relative risk of SEVERE ADVERSE EVENTS in the treatment group was 500% higher (.85%/.169%) than placebo.
More than .85% (15/1761) of all children who were injected with ModeRNA’s biological product suffered a SEVERE adverse reaction (versus .169% placebo)
Nearly 1.5% (26/1761) of children who were injected with ModeRNA’s biological product suffered an adverse event that required medical attention.
If 10 million young children were to receive these injections one could project that nearly 85,000 children would suffer SEVERE adverse events and nearly 150,000 children would suffer adverse events that would require medical attention.
SOURCE:
https://www.fda.gov/media/159157/download (page 94)
SOURCE:
https://www.fda.gov/media/159157/download (page 95)
SOURCE:
https://www.fda.gov/media/159157/download (page 97)
SOURCE:
https://www.fda.gov/media/159157/download (page 93)
SOURCE:
https://www.fda.gov/media/159157/download (page 90)
SOURCE:
https://www.fda.gov/media/159157/download (page 88)
SOURCE:
https://www.fda.gov/media/159157/download (page 88)
SOURCE:
https://www.fda.gov/media/159157/download (page 89)
SOURCE:
https://www.fda.gov/media/159157/download (page 90)
For additional information, please read this article:
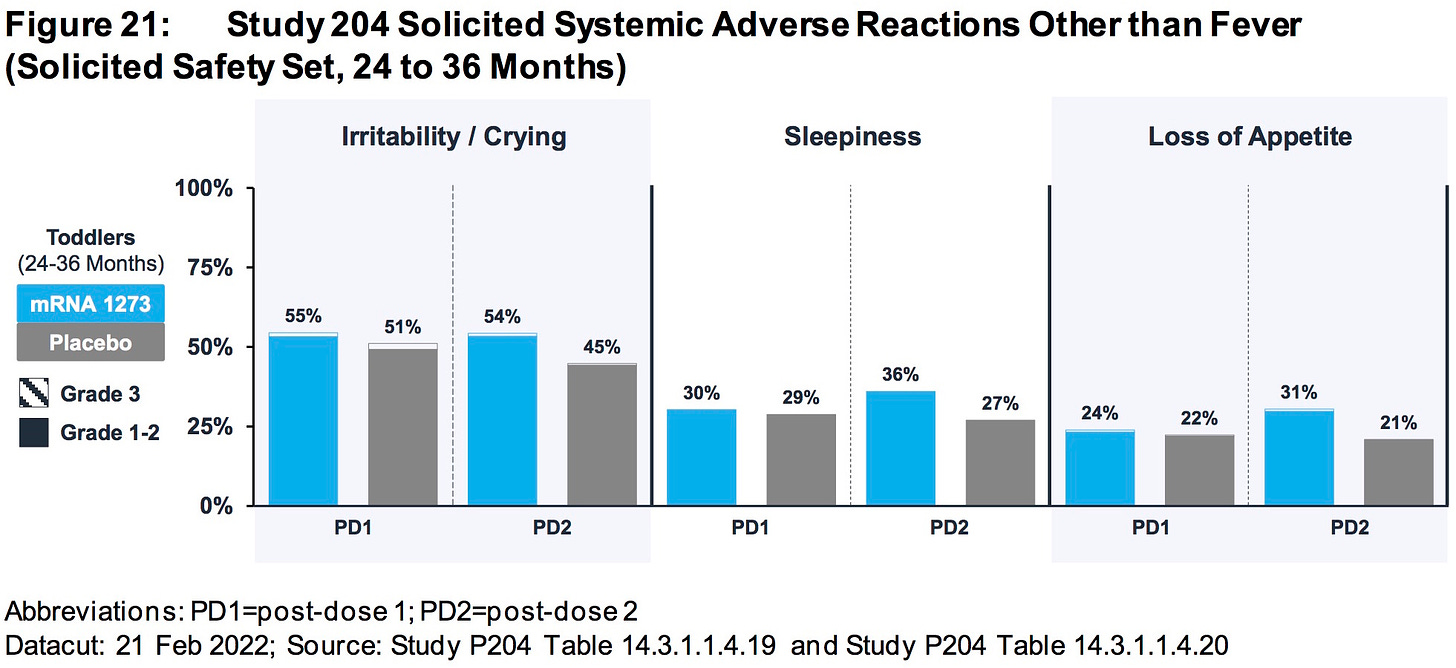
AGES 24-36 MONTHS:
SOURCE:
https://www.fda.gov/media/159157/download (page 78)
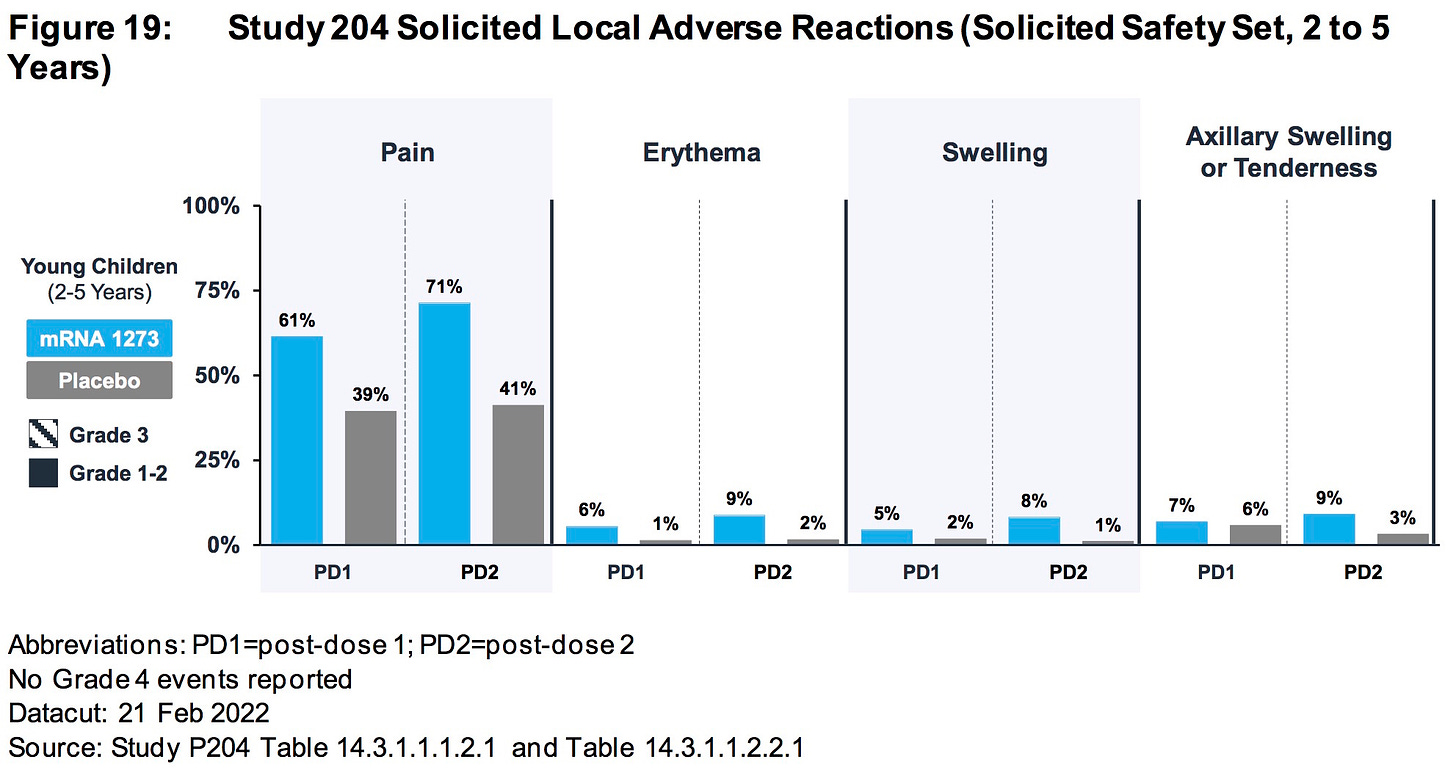
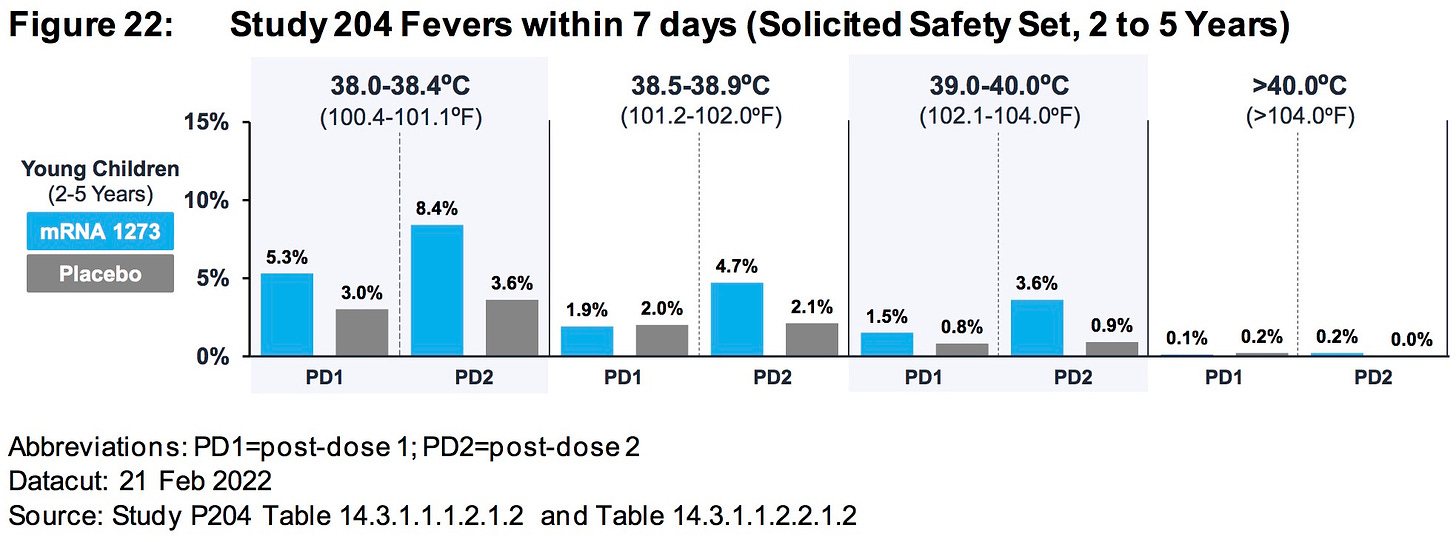
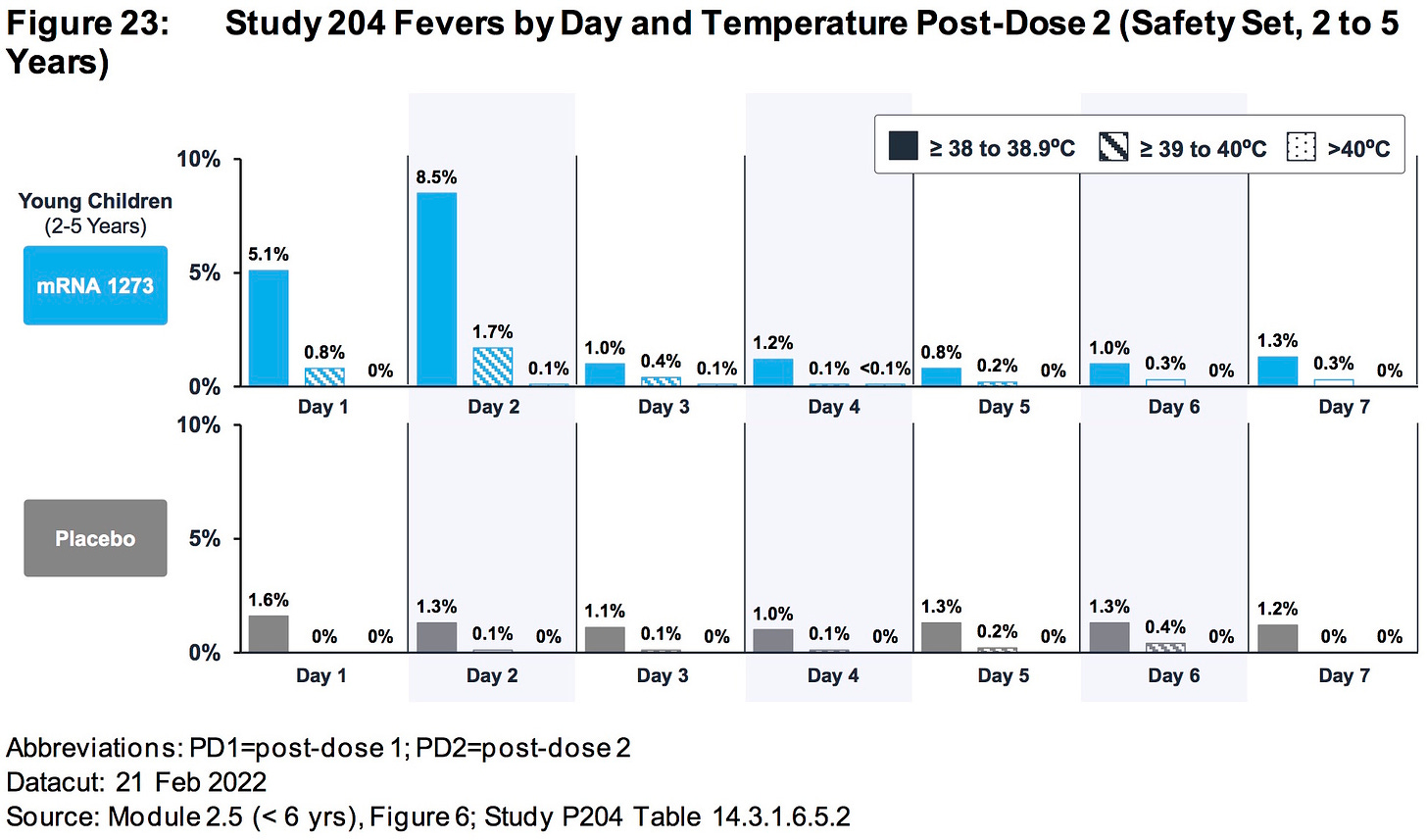
AGES 2 TO 5 YEARS:
Differences in rates of RSV infection (0.4% mRNA1273, < 0.1% placebo) and pneumonia (0.3% mRNA-1273, 0% placebo) were observed.
https://www.fda.gov/media/159157/download (page 81)
SOURCE:
https://www.fda.gov/media/159157/download (page 83)
SOURCE:
https://www.fda.gov/media/159157/download (page 84)
SOURCE:
https://www.fda.gov/media/159157/download (page 77)
SOURCE:
https://www.fda.gov/media/159157/download (page 79)
SOURCE:
https://www.fda.gov/media/159157/download (page 79)
AGES 37 MONTHS TO 5 YEARS:
SOURCE:
https://www.fda.gov/media/159157/download (page 78)
Myocarditis/pericarditis
Remaining uncertainties related to vaccine-associated myocarditis/pericarditis include:
Incidence in the age group of 6 months through 4 years, for which there is no real-world data available for any mRNA COVID-19 vaccine, and risk specific to mRNA-1273 in ages 5-11 years
Risk after additional primary series or booster doses of the vaccine
Long-term sequelae and outcomes in affected individuals
Whether and to what extent subclinical cases might occur, and if so, the long-term
outcomes
Mechanism of pathogenesis of vaccine-associated myocarditis/pericarditis
Individual factors conferring increased risk for vaccine-associated myocarditis/pericarditis.
SOURCE:
https://www.fda.gov/media/159189/download (page 179-180)
4. THE RISKS FAR OUTWEIGH THE BENEFITS
https://www.fda.gov/media/159189/download (page 181-182)
https://www.fda.gov/media/159189/download (page 177)
FDA GUIDELINES:
3.2 FDA Guidance for Industry Related to COVID-19 Vaccines
An EUA allowing for rapid and widespread deployment of the vaccine to millions of individuals, including healthy people, would need to be supported by clear and compelling evidence of effectiveness and adequate safety follow-up to make a determination of favorable benefit-risk.
https://www.fda.gov/media/159189/download (page 22)
For an EUA to be issued for a vaccine… FDA must determine that the known and potential benefits outweigh the known and potential risks of the vaccine.
https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
(B) the known and potential benefits of the product, when used to diagnose, prevent, or treat such disease or condition, outweigh the known and potential risks of the product, taking into consideration the material threat posed by the agent or agents identified in a declaration under subsection (b)(1)(D), if applicable;
https://www.law.cornell.edu/uscode/text/21/360bbb-3
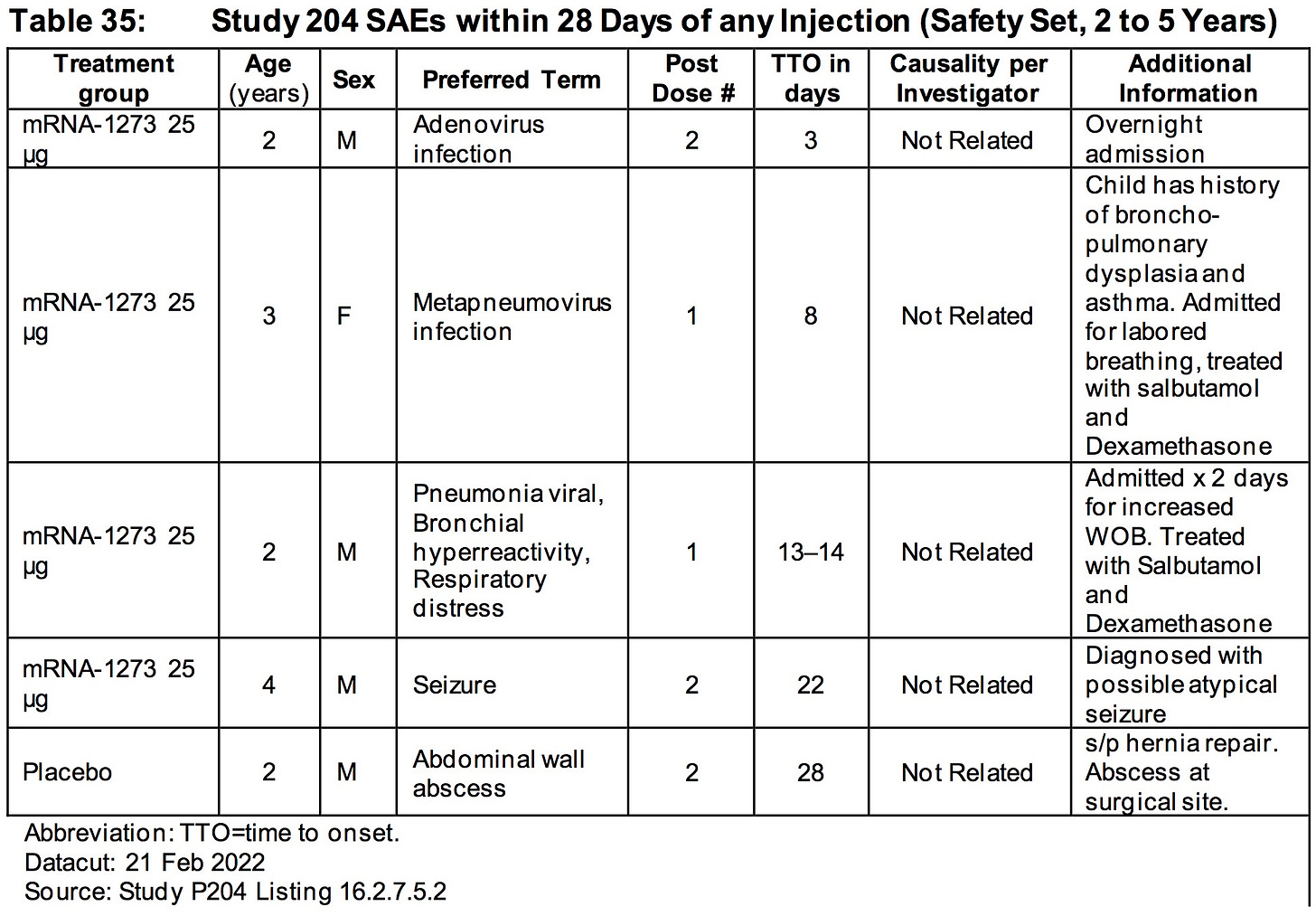
5. INADEQUATE TRIAL SIZE:
https://www.fda.gov/media/159189/download (page 178-179)
6. IMMUNOBRIDGING IS INNACCURATE, IMMORAL AND ILLEGAL
The ModeRNA document actually states the following….
The immune marker(s) used for immunobridging do not need to be scientifically established to predict protection
https://www.fda.gov/media/159189/download (page 21)
IT DOES NOT MATTER whether or not the FDA, the medical establishment or the scientific community accepts immunobridging as a proxy for true clinical proof of disease prevention because immunobridging is NOT LEGALLY APPROVED.
The law is clear. Immunobridging is NOT mentioned in the law, nor is it mentioned in current regulations, so it may NOT be used as a justification for approval until such time that either the law or the regulations have been changed to include it as a valid proxy.
The law states that the evidence must show that the biological product must be shown to PREVENT disease, not merely manipulate a proxy blood-borne immune system marker.
Under current law, the FDA does not have the authority to overrule the current legal requirement that a biological product be shown in clinical trials to PREVENT the disease in question. Proxy markers such as immunobridging are NOT mentioned in the law and may not legally be used as a justification to grant emergency use authorization.
SOURCE:
https://www.fda.gov/media/159189/download (page 10)
SOURCE:
https://www.fda.gov/media/159157/download (page 47)
SOURCE:
https://www.fda.gov/media/159189/download (pages 108-109)
READ THIS GREAT ARTICLE BY TOBY ROGERS:
No actual health benefits so Moderna/FDA use the immunobridging trick
The risks of Covid-19 are so low in the childhood population that there were ZERO severe cases of Covid-19 in either the treatment or the control group.
Therefore, the number needed to vaccinate, to prevent a single severe case of Covid-19 in the childhood population is infinity. (Technically it’s undefined because you cannot divide by zero, but you take my point). The FDA and CDC guidance documents for how to write a risk benefit assessment state that one must provide a number needed to treat, the absolute risk reduction, and the relative risk reduction. Moderna just skipped all that because the cartel makes its own rules.
Moderna is in a race against natural immunity. But natural immunity has already won because 74.2% of kids had natural immunity by February — so by now the number is probably closer to 100%. The God-given immune system in kids has already done its part to stop the pandemic and now the FDA wants to mess that up to enrich the cartel and keep the pandemic going forever.
So how does Moderna/FDA claim that this shot was “effective”? They use an unethical statistical trick called “immunobridging.”
It makes me mad that I even have to explain it because it’s such junk science. But we all need to know exactly how the FDA rigged the process so that we can explain to the jury at Nuremberg 2 why these monsters should be convicted so here goes:
Remember, the Moderna shots produced NO reductions in severe outcomes because the risk of Covid-19 in this age group is infinitesimally small (see studies: here, here, here, and here). So Moderna ignored the actual health outcomes and switched to looking at antibodies in the blood. In the process, they engaged in two egregious sleights of hand:
First, Moderna claims that the sample size for each of the four subgroups of children is about 3,000. But when it came to looking at antibodies in the blood, Moderna threw out about 90% of the sample and only looked at the bloodwork of about 300 kids in each age group. No explanation was given for the criteria they used to exclude 90% of the sample from their analysis. We know that up to 30% of kids have no antibody response at all to Covid-19 shots so perhaps they actually started with a much larger sample and then threw out the data that showed no effect from the shot?
The second sleight of hand is that “no placebo recipients were included in the Immunogenicity Subset” (p. 26). Do you realize how huge this is? This is no longer an RCT at all — they did not include the bloodwork from anyone in the placebo group. So the study cannot rule out the possibility that the increase in antibody levels was not from the vaccine at all but could have been from natural immunity. Just astonishing.
After these sleights of hand, Moderna then compares the antibody levels in the blood of about 10% of the children against the antibody levels in a sample of about 300 adults ages 18 to 25 enrolled in a previous clinical trial. If the antibody levels are similar (which they are), Moderna claims, ‘And therefore it will prevent disease in the future in kids!’
A few problems with that claim:
The Moderna study only measured antibody levels two months after the second dose — the time period when the antibody levels are at their peak (what Berenson calls “the happy valley”). But real world experience with these vaccines shows that any efficacy quickly wanes to zero by six months and then goes NEGATIVE after that.
The second problem, and this is unresolvable and instantly disqualifying for Moderna, is that at the April 6, 2022, meeting of the FDA’s “expert advisory committee” one member after another acknowledged that there are no “correlates of protection” for these vaccines. What that means in plain English is that you cannot use antibodies (or B-cells, T-cells, or any other proxy) to predict whether someone is immune or not.
Eric Rubin, who serves on that committee and is also the editor of the NEJM stated it bluntly, “We know what kind of antibody response can be generated, we just don’t know if it works.” You can watch it yourself on video:
The third problem is that the Moderna study was completed back in mid-2021 — when the original Wuhan and Alpha strains were prevalent. Since then, the Omicron variant has entirely replaced the original strains and real world data show that both Moderna and Pfizer shots are not effective against the Omicron variant. So in spite of all of the chicanery (discarding 90% of the sample, immunobridging, claiming correlates of protection that are not valid) Moderna cannot show any evidence that this shot will be effective against SARS-CoV-2 as it exists now.
7. QUALITY CONTROL FAILURES
Foreign substance detected in Moderna vaccine in Japan may be metal
https://www.japantimes.co.jp/news/2021/08/27/national/moderna-contamination-metal/
The FDA has failed miserably in its oversight requirements in regards to quality control, and good manufacturing practices.
Part of FDA’s evaluation of an EUA request for a COVID-19 vaccine includes evaluation of the chemistry, manufacturing, and controls information for the vaccine. Sufficient data should be submitted to ensure the quality and consistency of the vaccine product. FDA will use all available tools and information, including records reviews, site visits, and previous compliance history, to assess compliance with current good manufacturing practices.
SOURCE:
https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
8. STUDY BASICS:
OUTCOME MEASURES
Primary Outcome Measures :
Number of Participants with Solicited Local and Systemic Adverse Reactions (ARs) [ Time Frame: Up to Day 156 (7 days after each injection) ]
Number of Participants with Unsolicited Adverse Events (AEs) [ Time Frame: Up to Day 177 (28 days after each injection) ]
Number of Participants with Medically-Attended AEs (MAAEs) [ Time Frame: Up to Day 514 (1 year after booster dose) ]
Number of Participants with Serious Adverse Events (SAEs) [ Time Frame: Up to Day 514 (1 year after booster dose) ]
Number of Participants with Adverse Events of Special Interest (AESIs), Including Multisystem Inflammatory Syndrome in Children (MIS-C), Myocarditis and/or Pericarditis [ Time Frame: Up to Day 514 (1 year after booster dose) ]
Number of Participants with AEs Leading to Discontinuation From Study Post-Booster Dose Through the Last Day of Study Participation [ Time Frame: Day 149 (booster dose Day 1) through the last day of study participation (Day 514) ]
Number of Participants with Serum Antibody Levels that Meet or Exceed the Threshold of Protection From COVID-19 [ Time Frame: Day 57 (1 month after second injection) ]Threshold of protection as predefined for study.
Geometric Mean (GM) Value of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Specific Serum Antibody [ Time Frame: Day 57 (1 month after second injection) ]
Seroresponse Rate of Vaccine Recipients [ Time Frame: Day 57 (1 month after second injection) ]
GM Value of Post-Booster Dose SARS-CoV-2 Specific Serum Antibody [ Time Frame: Day 149 (post third dose) ]
Seroresponse Rate of Post-Booster Dose of Vaccine Recipients [ Time Frame: Day 149 (post third dose) ]
Secondary Outcome Measures :
GM Value of SARS-CoV-2 S-Protein-Specific Binding Antibody (bAb) [ Time Frame: Day 1, Day 57, Day 209, Day 394, booster dose Day 1, booster dose Day 29, booster dose Day 181, and booster dose Day 366 ]
GM Value of SARS-CoV-2- Specific Neutralizing Antibody (nAb) [ Time Frame: Day 1, Day 57, Day 209, Day 394, booster dose Day 1, booster dose Day 29, booster dose Day 181, and booster dose Day 366 ]
Number of Participants with SARS-CoV-2 Infections Regardless of Symptomatology, as Assessed by Serology and/or Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) [ Time Frame: Up to Day 394 ]Clinical signs indicative of SARS-CoV-2 infection as predefined for the study.
Number of Participants with SARS-CoV-2 Infection Measured by RT-PCR and/or bAb Levels Against SARS-CoV-2 Nucleocapsid Protein in Participants with Negative SARS-CoV-2 at Baseline, in the Absence of Any COVID-19 Symptoms [ Time Frame: Up to Day 394 ]
Number of Participants with a First Occurrence of COVID-19 [ Time Frame: Up to Day 394 ]Clinical signs indicative of COVID-19 as predefined for the study.
9. THE LAW IS CLEAR:
21 U.S. Code § 360bbb–3 - Authorization for medical products for use in emergencies
(c) Criteria for issuance of authorization
The Secretary may issue an authorization under this section with respect to the emergency use of a product only if, after consultation with the Assistant Secretary for Preparedness and Response, the Director of the National Institutes of Health, and the Director of the Centers for Disease Control and Prevention (to the extent feasible and appropriate given the applicable circumstances described in subsection (b)(1)), the Secretary concludes—
(1) that an agent referred to in a declaration under subsection (b) can cause a serious or life-threatening disease or condition;
(2) that, based on the totality of scientific evidence available to the Secretary, including data from adequate and well-controlled clinical trials, if available, it is reasonable to believe that—
(A) the product may be effective in diagnosing, treating, or preventing—
(i)such disease or condition; or
(ii)a serious or life-threatening disease or condition caused by a product authorized under this section, approved or cleared under this chapter, or licensed under section 351 of the Public Health Service Act [42 U.S.C. 262], for diagnosing, treating, or preventing such a disease or condition caused by such an agent; and
(B) the known and potential benefits of the product, when used to diagnose, prevent, or treat such disease or condition, outweigh the known and potential risks of the product, taking into consideration the material threat posed by the agent or agents identified in a declaration under subsection (b)(1)(D), if applicable;
(3) that there is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating such disease or condition;
(4) in the case of a determination described in subsection (b)(1)(B)(ii), that the request for emergency use is made by the Secretary of Defense; and
(5)that such other criteria as the Secretary may by regulation prescribe are satisfied.
SOURCE:
https://www.law.cornell.edu/uscode/text/21/360bbb-3
10. TAKE ACTION:
PLEASE CONTACT THE FOLLOWING PEOPLE AND SHARE YOUR OPINIONS WITH THEM ASAP:
Archana.Chatterjee@RosalindFranklin.edu
Aux7@cdc.gov
CBERVRBPAC@fda.hhs.gov
DeanofPublicHealth@brown.edu
Doran.Fink@fda.hhs.gov
Hong.Yang@fda.hhs.gov
Huilee.Wong@fda.hhs.gov
JYLee@uams.edu
Janet.Woodcock@fda.hhs.gov
Jportnoy@cmh.edu
Leslie.Ball@fda.hhs.gov
OFFIT@email.chop.edu
Peter.Marks@fda.hhs.gov
RandyHawkins@cdrewu.edu
Richard.Forshee@fda.hhs.gov
Xavier.Becerra@HHS.gov
acip@cdc.gov
acohn@cdc.gov
adam.berger@nih.gov
anc0@cdc.gov
archana.chatterjee@rosalindfranklin.edu
ashane@emory.edu
asmonto@umich.edu
bgellin@rockfound.org
cmeissner@tuftsmedicalcenter.org
commissioner@fda.hhs.gov
david.kim@hhs.gov
erubin@hsph.harvard.edu
erubin@nejm.org
fdaoma@fda.hhs.gov
fullerao@umich.edu
gcsylvester@gmail.com
hagans@stanford.edu
hanae@bcm.edu
hbernstein@northwell.edu
hgans@stanford.edu
hjanes@fredhutch.org
jportnoy@cmh.edu
mew2@cdc.gov
mhsawyer@ucsd.edu
mlevine@som.umaryland.edu
mrn8d@virginia.edu
ofer.levy@childrens.harvard.edu
officeofthepresident@mmc.edu
offit@chop.edu
paul.spearman@cchmc.org
paula.annunziato@merck.com
reingold@berkeley.edu
sean.mccluskie@hhs.gov
spergam@fredhutch.org
stanley-perlman@uiowa.edu
swamy002@mc.duke.edu
wayne_marasco@dfci.harvard.edu
11. SOURCES:
https://www.fda.gov/media/159157/download
https://www.fda.gov/media/159189/download
12. Jumping the Gun: WTF?
The videos below were published before the vaccines were even considered for authorization. This is evidence of collusion and conspiracy to defraud.
June 3, 2022
by James Roguski
The old system is crumbling, and we must build its replacement quickly.
If you are fed up with the government, hospital, medical, pharmaceutical, media, industrial complex and would like to help build a holistic alternative to the WHO, then feel free to contact me directly anytime.
JamesRoguski.substack.com/about
JamesRoguski.substack.com/archive
310-619-3055


















































Contact me directly if you have any questions. 310-619-3055
James: You've done an amazing job compiling the evidence that Moderna has provided no basis for the FDA to legitimately approve the COVID vaccine for children. I would like to add a couple of other ways they have failed: 1. They have failed to prove that the vaccine is safer or more effective at preventing serious COVID-19 or any COVID-19 in children COMPARED TO THE ALTERNATIVES. We are all familiar with the use of Vitamin D, HCQ and IVM as effective prophylaxis. We are also aware of the data demonstrating safety. The vaccine has not been proven to be safer or more effective that these (or other) alternatives. 2. They have failed to provide evidence of safety and efficacy of any type DONE BY INDEPENDENT RESEARCHERS. Moderna data can not be admitted as legitimate evidence as they are inextricably biased towards their own data. They have no INDEPENDENTLY CONDUCTED RESEARCH demonstrating the safety and efficacy of their products, so they have failed in this way too.