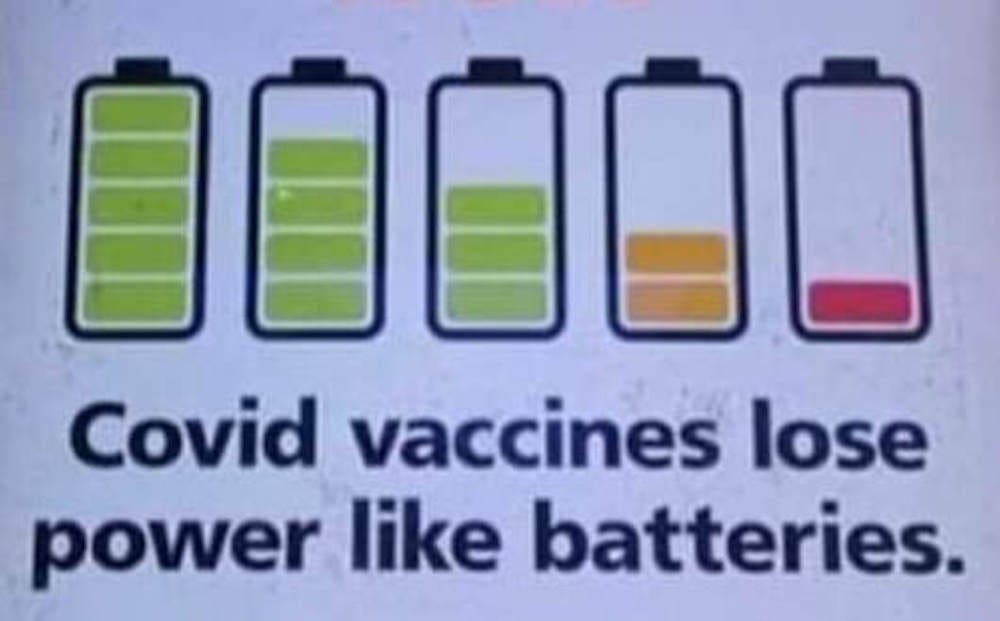
Evidence that the mRNA "Vaccines" are NOT effective
There is overwhelming evidence that the mRNA injections have failed miserably to perform as promised and cannot even be referred to as "vaccines" because they do NOT provide immunity.
FOR COMPLETE DETAILS: NotSafeAndNotEffective.com
How Pfizer “Presented” the Data in their Clinical Trial:
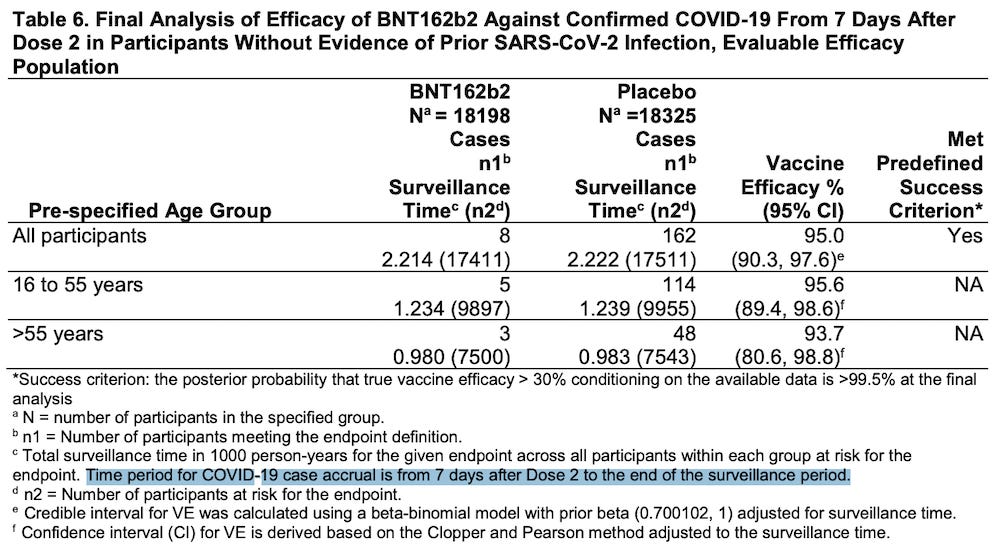
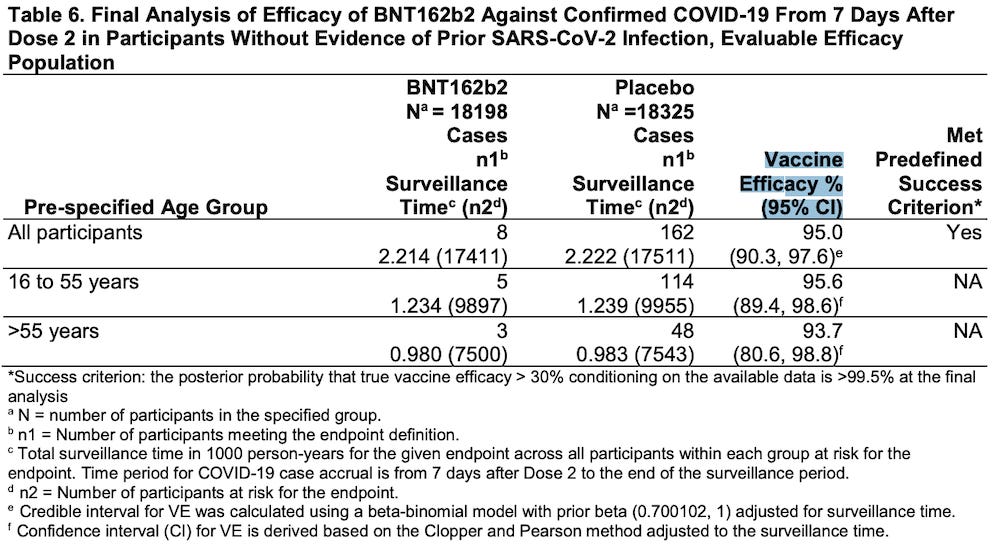
In their November 27, 2020 application for Emergency Use Authorization, Pfizer claimed that only 8/18,198 people who received the BNT162b2 “vaccine” were “diagnosed” with COVID-19.
Please read footnote “c” in Table 6 below.
https://www.fda.gov/media/144416/download (page 23)
The time period of the data collection period started “from 7 days after Dose 2.”
This means that for 28 days after receiving the first injection (21 days between injection #1 and injection #2, plus 7 days after Dose 2) any participant who had symptoms of COVID-19 was NOT INCLUDED IN THE CALCULATIONS OF VACCINE EFFICACY.
Please watch the video below:
https://rumble.com/v64z4ts-how-pfizer-manipulated-the-data-in-their-clinical-trial.html
KEY POINT:
Pfizer IGNORED all the data from the first 4 weeks of the study in calculating their claim of 95% efficacy.
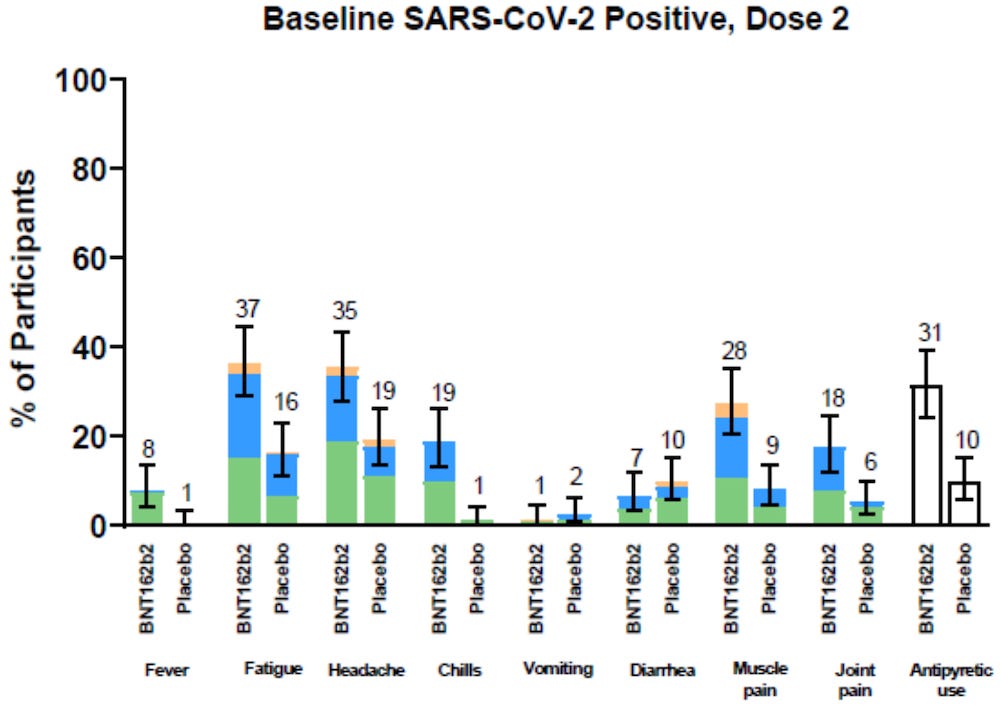
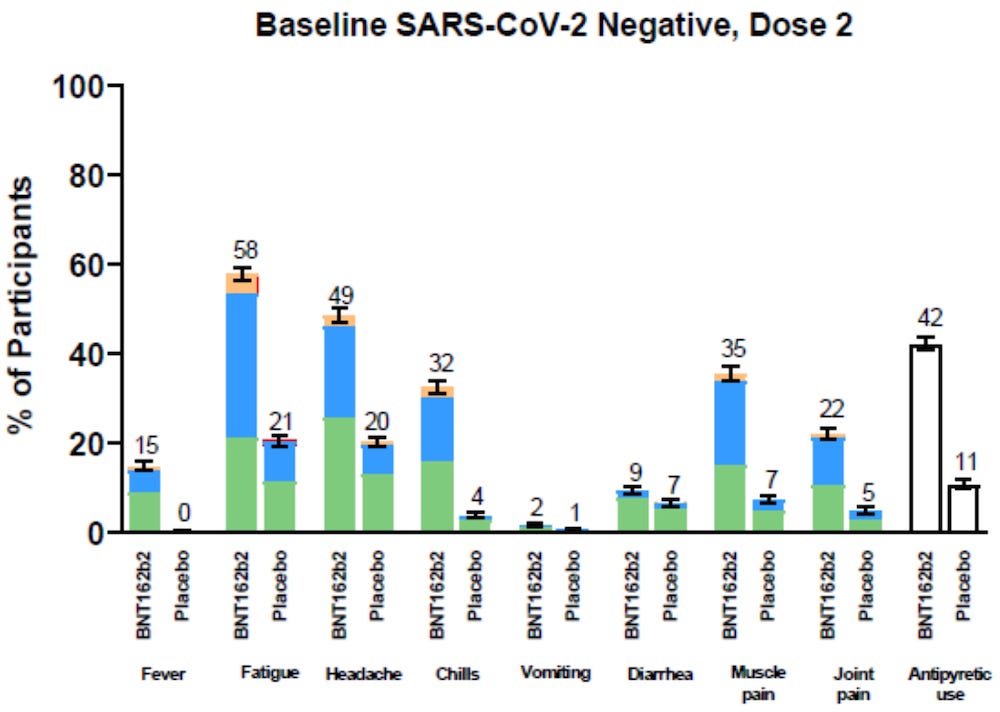
In the 4th week of the study alone, it was clear that those who received the Pfizer “vaccine” experienced far more symptoms of COVID-19 than those who received the placebo.
https://www.nejm.org/doi/suppl/10.1056/NEJMoa2110345/suppl_file/nejmoa2110345_appendix.pdf
Pfizer ignored the data above
Pfizer used the data below
Starting in week 5 and running until the end of the data collection period, Pfizer actually claims that only 8 people in the treatment group and 162 people in the placebo group were “diagnosed” with COVID-19.
Pfizer claims that only 8 out of 18,198 people in the treatment group were “diagnosed” with COVID-19, when the charts listed above clearly showed that the treatment group had vastly more COVID-19 symptoms than the placebo group in week 4.
The data below is what Pfizer uses to claim 95% efficacy?
REALLY?
REALLY?
https://www.fda.gov/media/144416/download (page 23)
It is unclear who was, or was not, given PCR “tests” in order to arrive at the final diagnosis of “COVID-19” that was used to determine the claim of 95% efficacy.
That’s another story!
PCRfraud.com
Pfizer’s cumulative analysis of post authorization adverse events report
https://phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf
Pfizer’s adverse events in detail
https://www.globalresearch.ca/wp-content/uploads/2023/05/pfizer-report.pdf
Transmission was NOT studied. (Watch the video below.)
https://x.com/Rob_Roos/status/1579759795225198593
Dr. Joseph Ladapo
https://www.bitchute.com/video/DKPJqkwZdezW/
Dr. Kevin Stillwagon
https://rumble.com/v5fp819-lies-and-truth-bombs.html
Israeli/ UK Data Show That The Controversial Vaccines Increase Prevalence Of Variants & Death Rates
REAL LIFE DATA ON INEFFECTIVENESS
December 6, 2021
552 Fully Vaccinated Oregon Residents Died Of COVID-19, Half Received Pfizer Vaccine
More than 500 residents in Oregon have now died of COVID-19 despite being fully vaccinated against the virus as health officials continue to report over 2,000 new infections daily.
December 9, 2021
Waning Immunity after the [Pfizer] BNT162b2 Vaccine in Israel
Among persons 60 years of age or older, the rate of infection in the July 11-31 period was [1.6] higher among persons who became fully vaccinated in January 2021 (when they were first eligible) than among those fully vaccinated 2 months later, in March.
Among persons 40 to 59 years of age, the rate ratio for infection among those fully vaccinated in February (when they were first eligible), as compared with 2 months later, in April, was 1.7.
Among persons 16 to 39 years of age, the rate ratio for infection among those fully vaccinated in March (when they were first eligible), as compared with 2 months later, in May, was 1.6
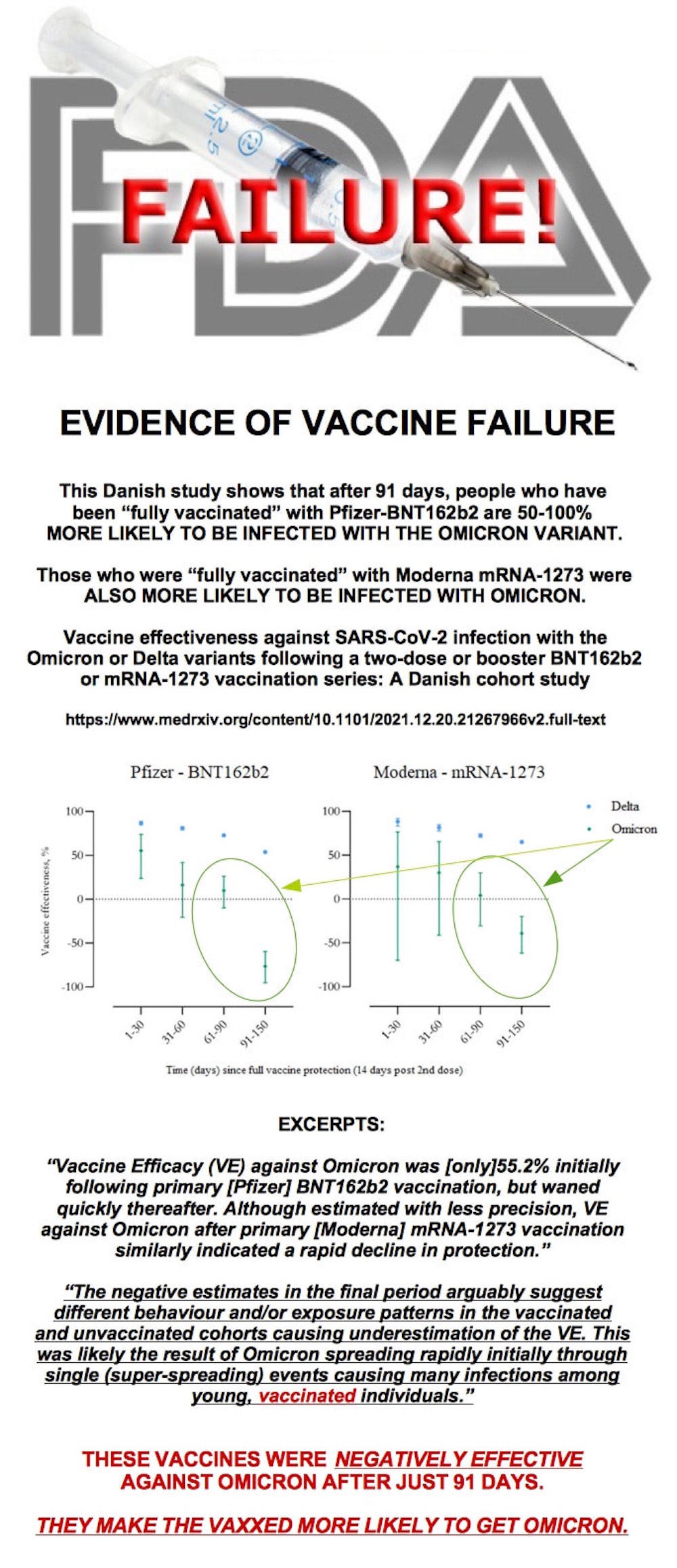
December 20, 2021 (Version 2)
Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study
Our study provides evidence of protection against infection with the Omicron variant after completion of a primary vaccination series with the BNT162b2 or mRNA-1273 vaccines; in particular, we found a Vaccine Effectiveness (VE) against the Omicron variant of 55.2% (95% confidence interval (CI): 23.5 to 73.7%) and 36.7% (95% CI: -69.9 to 76.4%) for the [Pfizer] BNT162b2 and [Moderna] mRNA-1273 vaccines, respectively, in the first month after primary vaccination.
However, the Vaccine Effectiveness (VE) is significantly lower than that against Delta infection and declines rapidly over just a few months.
https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v2.full
https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v2.full.pdf
December 23, 2021 (Version 3)
https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v3
https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v3.full.pdf
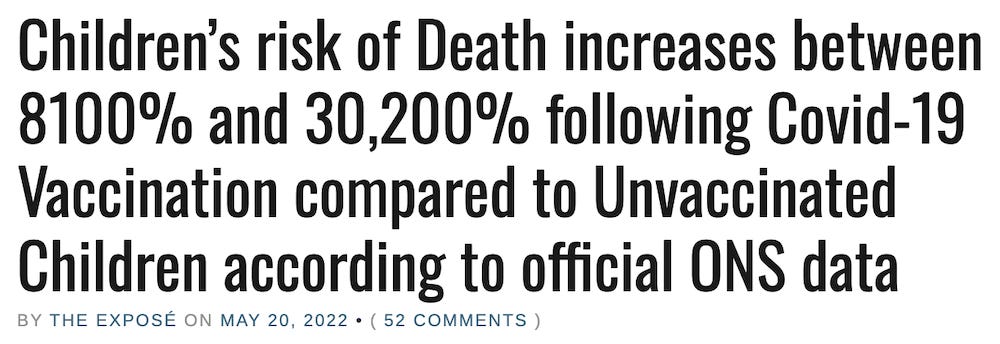
May 20, 2022
https://expose-news.com/2022/05/20/kids-death-risk-increases-8100percent-covid-vaccination/
June 14, 2022
Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance
At 2 to 4 weeks after dose 2,
among children, estimated Vaccine Effectiveness: 60.1%
among adolescents, estimated Vaccine Effectiveness: 59.5% [95% CI, 44.3%-70.6%]).
During month 2 after dose 2,
among children, estimated Vaccine Effectiveness: 28.9%
among adolescents, estimated Vaccine Effectiveness: 16.6%
November 30, 2022
Why Do Vaccinated People Represent Most COVID-19 Deaths Right Now?
By April 2022, the United States Centers for Disease Control and Prevention (CDC) data show that about 6 in 10 adults dying of COVID-19 were vaccinated or boosted.
https://www.kff.org/policy-watch/why-do-vaccinated-people-represent-most-covid-19-deaths-right-now/
December 1, 2022
Estimated BNT162b2 Vaccine Effectiveness Against Infection With Delta and Omicron Variants Among US Children 5 to 11 Years of Age
The adjusted Vaccine Effectiveness (VE) of 2 doses against Omicron at less than 3 months was 39%, and at 3 months or more, it was -1%
December 17, 2022
Effectiveness of the Coronavirus Disease 2019 (COVID-19) Bivalent Vaccine
Summary: Among 51011 working-aged Cleveland Clinic employees, the bivalent COVID-19 vaccine booster was 30% effective in preventing infection, during the time when the virus strains dominant in the community were represented in the vaccine.
https://www.medrxiv.org/content/10.1101/2022.12.17.22283625v1.full.pdf
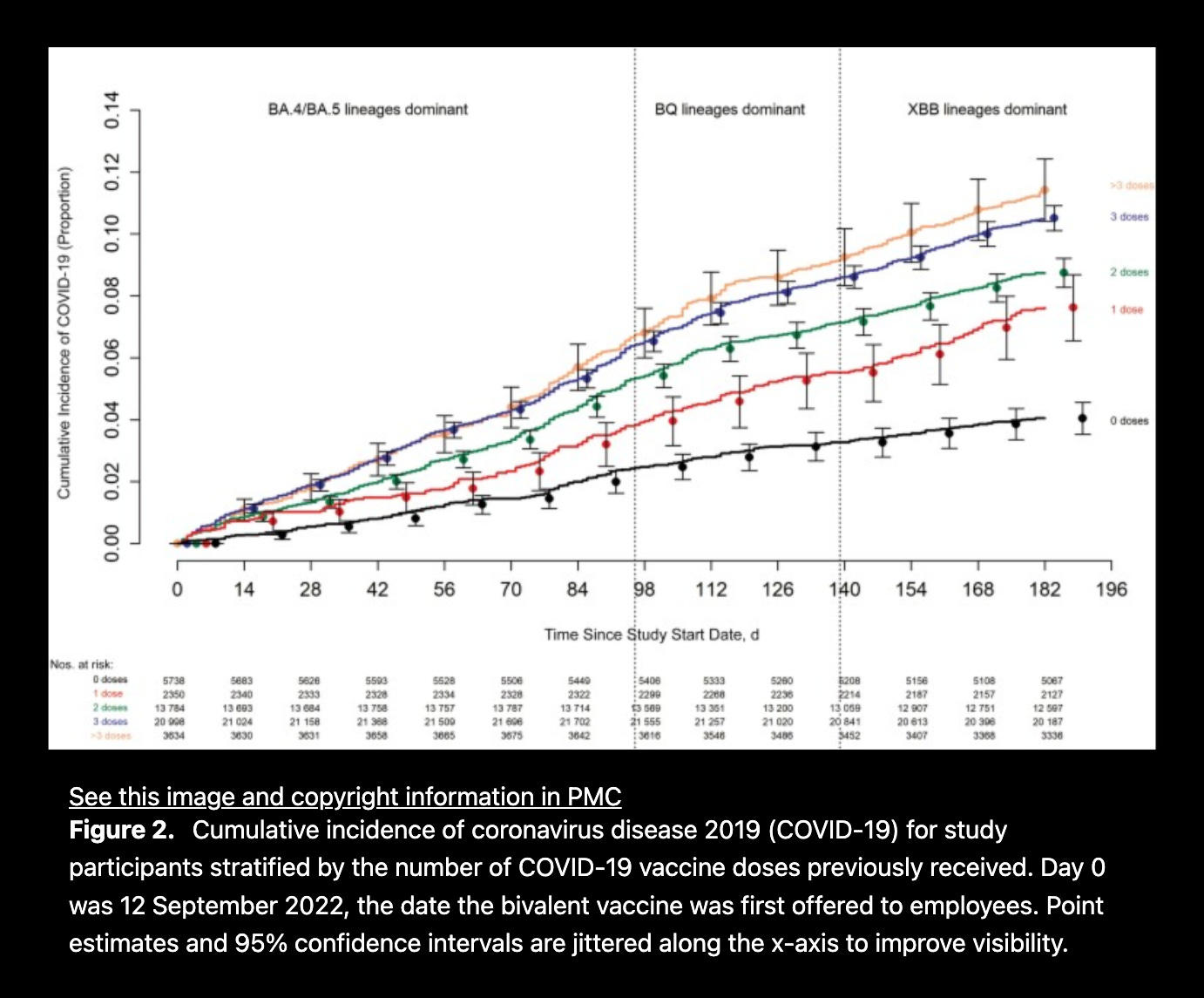
April 19, 2023
Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine
CLEVELAND CLINIC
The more injections people received, the more likely they were to be diagnosed with COVID-19.
The estimated vaccine effectiveness was
29% (21%-37%) during the BA.4/5 dominant phase
20% (6%-31%) during the BQ dominant phase
4% (-12% to 18%) during the XBB-dominant phase.
Decreased risk was not found during the XBB-dominant phase (0.96 [.82-.1.12]).
The risk of COVID-19 also increased with time since the most recent prior COVID-19 episode, and with the number of vaccine doses previously received.
April 29, 2023
Effectiveness of BNT162b2 Vaccine against Omicron Variant Infection among Children 5-11 Years of Age, Israel
Vaccine effectiveness estimates after the second vaccine dose were
58.1% for days 8-14,
53.9% for days 15-21,
46.7% for days 22-28,
44.8% for days 29-35, and
39.5% for days 36-42.
Vaccine effectiveness against Omicron infection among children 5-11 years of age… declined early and rapidly.
December 29, 2023
Infection with SARS-CoV-2 following Second Dose Pfizer-BioNTech mRNA COVID-19 Vaccine BNT162b2 in Danish Adolescents Aged 12-18 Years: A Real-World Nationwide Danish Cohort Study
During Omicron dominance, Vaccine Effectiveness (VE) was
5.8% in ages 12-15 years and
9.2% in ages 16-18 years
Thus, [Pfizer] BNT162b2-vaccine protection was limited during the Omicron era.
January 23, 2024
Effectiveness of COVID-19 Vaccines Over Time Against Clinical and Radiologic Outcomes Related to Severe SARS-CoV-2 Infection
Vaccine effectiveness against severe clinical outcomes and severe pneumonia related to SARS-CoV-2 infection gradually declined, with increased odds of both observed in patients vaccinated more than 240 days before diagnosis.
A Critical Analysis of All-Cause Deaths during COVID-19 Vaccination in an Italian Province
We found all-cause death risks to be even higher for those vaccinated with one and two doses compared to the unvaccinated and that the booster doses were ineffective. We also found a slight but statistically significant loss of life expectancy for those vaccinated with 2 or 3/4 doses.
July 1, 2024
COVID-19 Vaccine Effectiveness in Autumn and Winter 2022 to 2023 Among Older Europeans
Within 14 to 89 days after vaccination, seasonal COVID Vaccine Effectiveness (CVE) was 29% and relative COVID Vaccine Effectiveness (CVE) of second boosters was 34% against all SARS-CoV-2 variants.
Estimates decreased with time since vaccination, with no protection from 180 days after vaccination.
July 26, 2024
Real-world effectiveness of original BNT162b2 mRNA COVID-19 against symptomatic Omicron infection among children 5-11 years of age in Brazil: A prospective test-negative design study
The adjusted estimate of two-dose vaccine effectiveness against symptomatic Omicron was 3.1 %.
August 16, 2024
Effectiveness of the 2023-2024 Formulation of the COVID-19 Messenger RNA Vaccine
Estimated vaccine effectiveness was 42% before the JN.1 lineage became dominant, and 19% after.
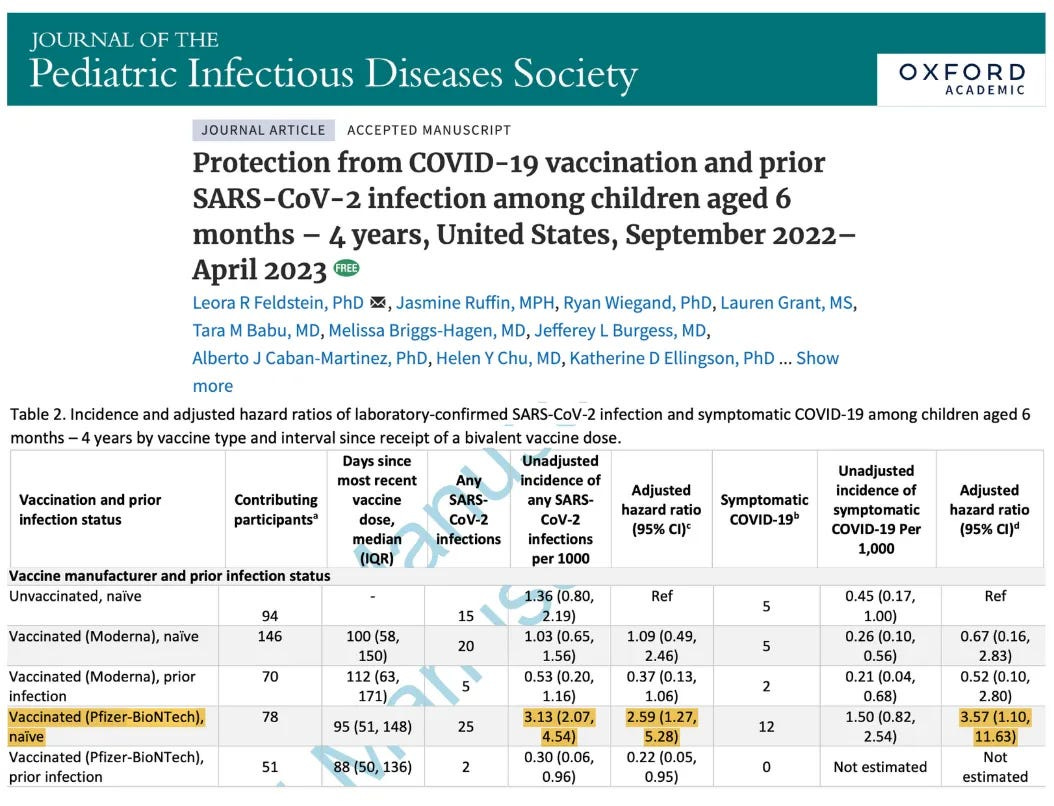
December 5, 2024
Protection from COVID-19 vaccination and prior SARS-CoV-2 infection among children aged 6 months - 4 years, United States, September 2022-April 2023
https://pubmed.ncbi.nlm.nih.gov/39656907/
https://academic.oup.com/jpids/advance-article/doi/10.1093/jpids/piae121/7917119?login=false
December 17, 2024
Evaluation of post-COVID mortality risk in cases classified as severe acute respiratory syndrome in Brazil: a longitudinal study for medium and long term
In the long term, comparing vaccinated and unvaccinated people, our study found that the risk of death can double in vaccinated people.
December 18, 2024
The Negative Efficacy of COVID-19 mRNA Injections Has Been Demonstrated
https://petermcculloughmd.substack.com/p/the-negative-efficacy-of-covid-19
“BREAKTHROUGH” INFECTIONS
What is a “breakthrough case?”
“breakthrough case” - an instance in which a person becomes sick with a disease despite having received the vaccine for that disease. In other words, the vaccine fails to prevent the person from becoming infected.
https://www.dictionary.com/e/tech-science/breakthrough-case/
Many thanks to Karen Kingston for pointing out the following information:
On page 29-30 of the FDA “Package Insert and FDA Approved Patient Labeling” for Pfizer’s COMIRNATY, their own document states that among those people participating in a subset from Study 5 “who had previously received a 2-dose primary series and 1 booster dose with COMIRNATY”
75.2% of the 105 participants 12-17 years of age were positive for SARS-CoV-2
71.7% of the 297 participants 18-55 years of age were positive for SARS-CoV-2
61.5% of the 286 participants 56 years of age and older were positive for SARS-CoV-2
PLEASE REALIZE WHAT THE ABOVE REALLY MEANS
In their own document, Pfizer is saying that in a group of people who had already been double vaxxed AND boosted, between 61.5% and 75.2% still tested positive for SARS-CoV-2.
https://www.fda.gov/media/151707/download (pages 29-30)
COVID-19 Vaccine Breakthrough Infections Reported to CDC — United States, January 1–April 30, 2021
A total of 10,262 SARS-CoV-2 vaccine breakthrough infections had been reported from 46 U.S. states and territories as of April 30, 2021. Among these cases, 6,446 (63%) occurred in females.
Based on preliminary data…
995 (10%) patients were known to be hospitalized, and 160 (2%) patients died.
The CDC stopped releasing data on “breakthrough cases” on May 1, 2021.
The agency will no longer regularly track and release the number of new COVID infections."
A very large collection of evidence on breakthrough cases can be found here:
https://covidindex.science/topics/breakthrough-cases
AFTER ACTION REVIEW OF THE COVID-19 PANDEMIC:
The Lessons Learned and a Path Forward
United States Congress’ Select Subcommittee on the Coronavirus Pandemic Committee on Oversight and Accountability
The COVID-19 vaccines are arguably more akin to treatments than the traditional vaccines the American public is used to receiving in early childhood. The mRNA vaccines for COVID-19 did not prevent human-to-human transmission nor prevent COVID-19 infection in the way that traditional vaccines have been able to do. Not fully and honestly explaining this dynamic was a critical public health messaging failure. (page 257)
Throughout the early rollout of COVID-19 vaccinations in the winter and spring of 2021, there was an aggressive and widespread campaign—often with the support of government public health institutions—to convince the American people to get vaccinated. However, the nuances of the vaccines’ regulatory status were unclear to most regular people. Instead, these novel mRNA vaccines were dubbed simply as “safe and effective,” with very little opportunity for patients to discuss these vaccines with their doctor and assess their individual risks and benefits. (page 348 in the PDF)
However, it was already evident then and is now commonly known that the vaccines do not prevent you from getting infected or transmitting the virus. (page 383 in the PDF)
https://docs.house.gov/meetings/VC/VC00/20241204/117748/HRPT-118-SSCPReport.pdf
COVID-19 Modified mRNA “Vaccines”: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio- Pharmaceutical Complex, Part 2
Disappointingly, now four years after the Registrational Trial reports that led to the rushed and massive worldwide “vaccination” campaign, not a single large controlled clinical trial has demonstrated that the modmRNA injections can reduce severe disease, hospitalization, and mortality. In light of this fact, the “safe and effective” mantra issued by the Bio-Pharmaceutical Complex throughout the pandemic must be deemed scientifically fallacious and moreover dangerous from a public health perspective.
Contrary to the pharmaceutical industry’s relentless messaging, three large well-designed cohort studies have demonstrated that repeated modmRNA injections lead to increased rates of COVID-19, with zero injections offering superior protection when compared to one or more injections (Shrestha et al., 2023; Shrestha et al., 2023b; Shrestha et al., 2024).
These findings are further reinforced by real-world observations of negative protection associated with COVID-19 modmRNA injections in various populations (UK Health Security Agency, 2022; COVID-19 Vaccine Surveillance Report, 2022; Eythorsson et al., 2022; Hatchard, 2022; Altarawneh et al., 2022; Gazit et al., 2022; Rzymski et al., 2021; Adhikari et al., 2024).
In November 2023, the CDC alarmingly inserted the COVID-19 modmRNA injections into the childhood immunization schedule, with a recommendation to have one dose of the updated or “current vaccine” administered to all children after six months of age (Cottrell, 2023). The recommendation is groundless and morally abhorrent, given that infants and children have only very rarely suffered from COVID-19, at rates that must be considered extremely minuscule when compared to the myocardial damage and other well-documented harms incurred by the modmRNA injections. Furthermore, no controlled clinical trials involving children have been conducted to adequately test the safety of the modmRNA “booster” products (in accordance with established scientific standards for either classical vaccines or gene-therapy products).
Virtually all children have now been exposed to the coronavirus and thus harbor enduring T-cell immunity (Turner et al., 2021; Patalon et al., 2023) that is vastly superior to any theoretical protection conferred by these untested modmRNA injectables (see Part 1). The harm-to-reward ratio may be incalculably large in light of the various cardiovascular, neurological, and malignant events (many of which may have a long latency) discussed in subsequent sections. Even if some of these conditions are indeed “rare” as many papers claim, given the vast numbers of these conditions and the likelihood that many disorders have a long latency, this would suggest a high harm-to- reward ratio for children and young adults. Data from a large pharmacovigilance study indicate a disproportionate reporting of immune-related adverse events among adolescents following the COVID-19 modmRNA injections (Kim et al., 2024a).
https://www.ijvtpr.com/index.php/IJVTPR/article/view/104/359
Hiding Evidence of “Vaccine” Failure
‘Vaccine breakthrough’ is a marketing term created by the CDC to describe what had previously been referred to as vaccine efficacy failure or simply, vaccine failure. Specifically, ‘vaccine breakthrough’ is a way to count how many people deemed to be ‘fully vaccinated’ still contracted the infection despite being ‘fully vaccinated’. In other words, ‘vaccine breakthrough’ establishes how many times the experimental COVID inoculations failed to prevent infection in the ‘fully vaccinated’ population and is a crucial metric for assessing the success or failure of the experimental COVID inoculations.
On April 30, 2021, with only four months of preliminary ‘vaccine breakthrough’ data collected from only a handful of states, CDC director, Defendant Walensky and HHS director, Defendant Becerra, authorized the CDC to stop the tracking and publication of ‘vaccine breakthrough’ cases.
Reporting on ‘vaccine breakthrough’ hospitalizations and deaths by the CDC would continue until October 30, 2021, when Defendant Walensky and Defendant Becerra would again authorize the CDC to stop the tracking and publication of all ‘vaccine breakthrough’ hospitalizations and deaths as ‘vaccine breakthrough’ hospitalizations and deaths began to mount despite only 28 state public health departments reporting this relevant metric. By the time of this decision to stop the tracking and publication of all ‘vaccine breakthrough’ data, it had become obvious that the existing experimental COVID inoculations were failing to prevent infection and severe symptomology in the ‘fully vaccinated’ population.
Collecting data from the 28 out of 51 state public health departments, including Washington D.C., reporting data on any aspects of ‘vaccine breakthrough’ the Petitioners learned the following:
• By November 2021, in the ‘fully vaccinated’ there were already well over 1.4 million confirmed infections, over 56,000 confirmed hospitalizations, and over 16,000 confirmed deaths.
• By December 2021, in the ‘fully vaccinated’ these numbers had increased exponentially to well over 2.5 million confirmed infections, over 90,000 confirmed hospitalizations, and almost 25,000 confirmed deaths in a single month.
• By January 2022, in the ‘fully vaccinated’ these numbers had increased exponentially yet again to well over 6.0 million confirmed infections, over 138,000 confirmed hospitalizations, and over 32,000 confirmed deaths in only a single month.
• By February 2022, in the ‘fully vaccinated’ these numbers had increased exponentially yet again to almost 9.0 million confirmed infections, over 191,000 confirmed hospitalizations, and almost 44,000 confirmed deaths in only a single month.
Despite the CDC and HHS decision to terminate reporting and the multitude of special rules put in place by the CDC to limit ‘vaccine breakthrough’ reporting, significant numbers of individuals have fallen ill, have been hospitalized and unfortunately lost their lives because an experimental product failed to protect them.36
Interestingly, ‘vaccine breakthrough’ data was replaced by Defendants Walensky and Becerra, using a new metric never used before referred to as ‘vaccine effectiveness’ that enables them to group unvaccinated persons, partially vaccinated persons, and fully vaccinated person with less than 14 days since the final shot in the series fraudulently as ‘unvaccinated’ persons. This was done to hyperinflate the data in the ‘unvaccinated’ group while simultaneously lowering the number of people in the ‘fully vaccinated’ group and paint a narrative picture to the public that what is occurring is a ‘pandemic of the unvaccinated’ which is completely unsubstantiated and flies in the face of data from around the world. The Defendants have promoted fraudulent data despite knowing through reports by the Department of Defense and the CDC as early as August 6, 2021, that ‘vaccine effectiveness’ to protect against infection in the ‘fully vaccinated’ wanes rapidly within months of inoculation.37
As of February 16, 2022, the CDC has not updated ‘vaccine effectiveness’ data to incorporate new data demonstrating exponential rises in ‘vaccine breakthrough’ since December 25, 2021. However, the CDC does confirm that their ‘vaccine effectiveness’ calculations are based upon only 28 state health departments reporting this relevant metric.38 (Please refer to Exhibit K for vaccine breakthrough data, citations, and images.)
Allegation: We allege Defendant Becerra and Defendant Walensky have intentionally acted to remove data from public view that could influence a citizen’s decision to decline the experimental COVID inoculations on the basis of failure to protect against infection. Removal of such information constitutes failure to satisfy Informed Consent laws governing the use of experimental medical products and codified as 45 CFR 46. The termination of this reporting removes key data essential for analysis of efficacy for products still in preliminary clinical trial until Oct 27, 2022 (Moderna/NIAID)39, Jan 2, 2023 (Johnson & Johnson)40, and May 15, 2023 (Pfizer/BioNTech)41 according to ClinicalTrials.gov. Where there is risk of injury, and risk of failure to prevent infection, there must always be informed consent with respect to human participation in ongoing clinical trials, including the use of EUA approved medical products with no long-term safety or efficacy data available.
The Defendants have violated informed consent by terminating all reporting for ‘vaccine breakthrough’ on October 30, 2021, in anticipation of an exponential increase ‘vaccine breakthrough’ cases, hospitalizations, and deaths. This act of withholding information from Americans while publishing fraudulent data intentionally to mislead and coerce Americans into the use of experimental products still in clinical trial is an egregious act of willful misconduct that places the lives of Americans in danger.
36 https://www.cdc.gov/coronavirus/2019-ncov/php/hd-breakthrough.html
37 https://www.cdc.gov/mmwr/volumes/70/wr/mm7031e2.htm
The mRNA "vaccines" have failed miserably to do what "vaccines" are supposed to do and they have fraudulently lied about that reality as evidenced by the information below.
FDA says a coronavirus vaccine would have to be at least 50% effective to be approved
FDA Commissioner Stephen Hahn addressed concerns about a fast-tracked approval process in a news release.
"While the FDA is committed to expediting this work, we will not cut corners in our decisions and are making clear through this guidance what data should be submitted to meet our regulatory standards,” Hahn said.
Report 02: Pfizer – 136 Deaths and 1625 Serious Cases of ‘Ineffectiveness’ Revealed
Beginning on December 1, 2020, Pfizer was aware that the vaccine that was pushed upon the American people had limited efficacy.
For the next 3 months, from 12/1/2020-2/28/2021, Pfizer’s 5.3.6 cumulative analysis of post authorization adverse events reports indicate that Pfizer received multiple reports of both vaccine failure and vaccine ineffectiveness.
In the same Pfizer document, Covid-19 is identified as an adverse event special interest (AESI), with 3,067 cases of Covid-19 reported after receiving the vaccine. From that number, there were 2,585 serious relevant events, including Covid-19 pneumonia, and 136 people died. (Page 17)
Pfizer excluded cases from analysis, including 546 cases in which SARS-CoV-2 infection was developed between days 1-13 from the first dose. (Page 15). After allowing for Pfizer’s exclusion of some cases, this data still reveals multiple serious cases, including fatalities, indicating there is vaccine failure and vaccine ineffectiveness with Pfizer’s vaccine.
And worse, Pfizer, who is responsible for the post authorization analysis, admits that there are limitations in the reporting and that “the magnitude of underreporting is unknown.” (Page 5).
According to Pfizer’s cumulative analysis, there were 16 serious cases of vaccine failure and 1,625 serious cases of vaccine ineffectiveness reported.
Even though there were multiple reports of lack of vaccine efficacy, Pfizer stated in the confidential document that “No new safety signals of vaccine lack of efficacy have emerged based on a review of these cases.” (Page 15)
https://dailyclout.io/pfizer-136-deaths-and-1625-serious-cases-of-ineffectiveness-revealed/
Report 34: Understanding C-19 Vaccine Efficacy Clinical Trial in Lay Terms
Moderna vaccination in people that have never had COVID previously reduces the production of anti-nucleocapsid antibodies compared to placebo. This may reduce the strength and duration of immunity to COVID compared to unvaccinated immune responses. The more doses, the less the production of anti-nucleocapsid antibodies. The Moderna vaccine decreases the production of antibodies to the nucleocapsid in a dose dependent fashion in those who acquire COVID after vaccination.
Four months after injection, 40% of vaccinated participants who acquired COVID after the second injection produced antibodies to the nucleocapsid, compared to 93% of those who received placebo injections.
In participants that were COVID positive on the day of Dose 1 injections (before the vaccinations had time to work) a robust production of anti-nucleocapsid antibodies occurred in both placebo and vaccinated groups, with no difference between the groups.
In the participants that acquired COVID between doses, a reduction in anti-nucleocapsid antibodies was observed in those who received the vaccine compared to those who received placebo. The reduction was not as severe as the group who acquired COVID after the second dose. Thus, it appears the more doses received, the more severe the reduction in anti-nucleocapsid antibody production.
If the mRNA vaccines decrease the production of anti-nucleocapsid antibodies in a dose dependent fashion, immunity would be short-lived and possibly lessened with additional boosters, the opposite of the desired outcome.
https://dailyclout.io/understanding-c-19-vaccine-efficacy-clinical-trial-in-lay-terms/
The Pfizer clinical trial actually demonstrated “negative effectiveness,” but that portion of the data was ignored.
The COVID-19 vaccines are the miracle that wasn’t. At the end of 2020, Defendant Pfizer, Inc. (Defendant or Pfizer) broadcast to the world that its COVID-19 vaccine was “95% effective.” Based on this and other statements made by Pfizer touting the efficacy of its new vaccine, Americans were given the impression that Pfizer’s vaccine would end the coronavirus pandemic and lift the omnipresent veil of fear and uncertainty from an anxious public. Placing their trust in Pfizer, hundreds of millions of Americans lined up to receive the vaccine. Contrary to Pfizer’s public statements, however, the pandemic did not end; it got worse. More Americans died in 2021, with Pfizer’s vaccine available, than in 2020, the first year of the pandemic. This, in spite of the fact that the vast majority of Americans received a COVID-19 vaccine, with most taking Pfizer’s. Indeed, by the end of 2021, official government reports showed that in at least some places a greater percentage of the vaccinated were dying from COVID-19 than the unvaccinated. Pfizer’s vaccine plainly was not “95% effective.” (page 1)
While Pfizer’s 95% figure made its vaccines seem highly effective, the truth was quite different. When it began making those claims, Pfizer possessed on average only two months of clinical trial data from which to compare vaccinated and unvaccinated persons. Of 17,000 placebo recipients, only 162 acquired COVID-19 during this two-month period. Based on those numbers, vaccination status had a negligible impact on whether a trial participant contracted COVID-19. The risk of acquiring COVID-19 was so small in the first instance during this short window that Pfizer’s vaccine only fractionally improved a person’s risk of infection. (page 2)
The State has reason to believe that Pfizer is engaging in or has engaged in the unlawful acts or practices set forth below. In addition, the State has reason to believe that Pfizer has caused injury, loss, and damage to it, as well has caused adverse effects to the lawful conduct of trade and commerce, thereby directly or indirectly affecting the people of this State. (page 6)
Pfizer designed the trial such that “defined COVID-19 cases” were counted starting only seven days after a participant received the second of two shots (at least 28 days after the first shot).
Put differently, COVID-19 cases that occurred before that point—that is, between shot one and seven days after shot two— were not considered when evaluating the efficacy of Pfizer’s vaccine.
That was a highly significant qualifier because 409 “[s]uspected” COVID-19 cases occurred after the participant received the first vaccine shot, but before seven days elapsed after taking the second shot. By contrast, only 287 suspected COVID-19 cases occurred among placebo recipients in that same interval.
In other words, more people in the trial’s treatment group experienced COVID-19 than in the placebo group, even though the former had taken at least one ostensibly immunity enhancing dose. (pages 18-19)
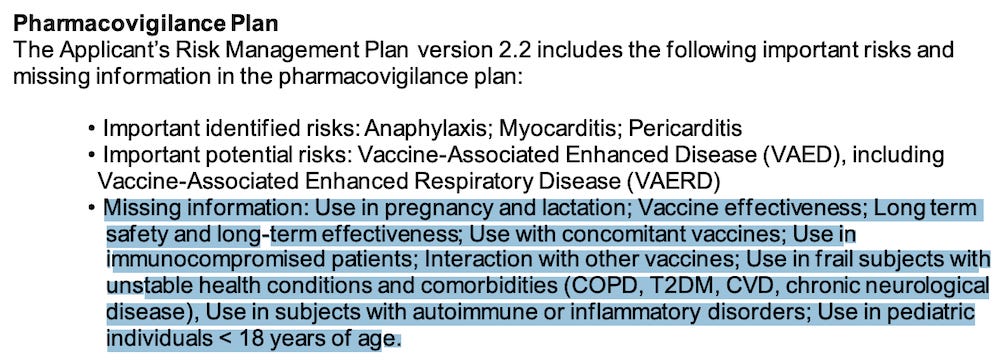
The FDA’s own documents clearly show that “effectiveness” has not been established and that a lot of important information is “missing.”
Package Insert and FDA Approved Patient Labeling - COMIRNATY (Pfizer)
Summary Basis for Regulatory Action (11/8/2021) COMIRNATY (Pfizer)
Summary Basis for Regulatory Action (1/30/2022) SPIKEVAX (Moderna)
Relative Risk Reduction versus Absolute Risk Reduction
Risk ratios are widely misused in ways that exaggerate both the benefits and harms of drugs. This is especially true regarding the term “relative risk reduction”.
Despite the fact that “relative risk reduction” is commonly cited, it does not really measure “risk” at all.
If a drug changes risk from 2 deaths per 10 people to 1 death per 10 people, the relative risk reduction (50%) is the same as if the drug changes risk from 2 deaths per 1,000,000 people to 1 death per 100,000,000 people.
Saving 1 out of every 10 lives is obviously different than saving 1 out of every 100,000,000 lives so, while it may be accurate, it is deceptive to say that both of these changes represent a 50% “relative risk reduction.”
Absolute risk reduction must be the primary consideration.
Misrepresentations concerning 95% relative risk reduction
Pfizer’s press release nowhere mentioned or explained the distinction between absolute and relative risk reduction, much less disclosed the fact that the misleading statements in its marketing promotion press release were based on relative risk reduction or that the absolute risk reduction equaled only 0.85%. At bottom, however, Pfizer concealed and, ultimately, never informed the public of the highly material fact that amongst the general population Pfizer’s own trial results showed that the vaccine would reduce the incidence of the non-vaccinated contracting COVID-19 by less than one percent.
Moreover, Pfizer’s data in its EUA submission that purported to answer that question could do so only for a limited period of time (two months after the second dose).
Taken alone and in combination, Pfizer’s misleading statements created the false impression that 95% of vaccine recipients would never obtain COVID-19, full stop.
Pfizer’s misrepresentations extended well beyond this 95% efficacy statement.
Over the following year, Pfizer would go on to mislead the public across multiple critical COVID- 19-related dimensions, including specifically the ability of the vaccine to prevent viral transmission from asymptomatic to uninfected people, the reality of waning vaccine efficacy, and the vaccine’s ineffectiveness against the Delta variant.
https://www.texasattorneygeneral.gov/sites/default/files/images/press/Pfizer%20Vaccine%20Petition%20Filed.pdf (page 21-24)
How the adverse effect counting window affected vaccine safety calculations in randomised trials of COVID-19 vaccines
Issues with counting windows could mean that there are no net benefits, and possibly even net deficits.
Risk of Coronavirus Disease 2019 (COVID-19) among those up-to-date and not up-to-date on COVID-19 vaccination by US CDC criteria
Conclusions: Since the XBB lineages became dominant, adults "up-to-date" on COVID-19 vaccination by the CDC definition do not have a lower risk of COVID-19 than those "not up-to-date", bringing into question the value of this risk classification definition.
Covid-19: Fully vaccinated people can carry as much Delta virus as unvaccinated people, data indicate
https://www.bmj.com/content/374/bmj.n2074
https://pubmed.ncbi.nlm.nih.gov/34413020/
Very few children 5-11 years of age were diagnosed with COVID-19.
Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age
[“A total of 255,936 children were included in the analysis. Among unvaccinated children, the crude incidence rates of all reported SARS-CoV-2 infections, PCR-confirmed SARS-CoV-2 infections, and Covid-19-related hospitalizations were 3303.5, 473.8, and 30.0 per 1 million person-days”]
Articles from Children’s Health Defense
https://childrenshealthdefense.org/defender/media-vaccine-safety-dr-stanley-plotkin-study/
https://childrenshealthdefense.org/defender/cdc-redact-myocarditis-information-foia-covid-shots/
https://childrenshealthdefense.org/defender/canada-dna-contamination-pfizer-covid-vaccine/
https://childrenshealthdefense.org/defender/covid-vaccines-dna-contamination/
https://childrenshealthdefense.org/defender/pfizer-biontech-covid-vaccine-placebo/
https://childrenshealthdefense.org/defender/confidential-eu-documents-deaths-pfizer-biontech-shots/
https://childrenshealthdefense.org/defender/switzerland-stops-covid-vaccines-immunity/
https://childrenshealthdefense.org/defender/aseem-malhotra-bbc-suspension-mrna-vaccines/
https://childrenshealthdefense.org/defender/women-covid-vaccine-injuries-v-safe-data/
https://childrenshealthdefense.org/defender/denmark-covid-campaign/
James Roguski
310-619-3055
JamesRoguski.substack.com/archive
ControlBloodSugarNaturally.com
I claim no copyright of any kind whatsoever, over any of my work, ever. Everyone is encouraged to copy any and all of it, in part, or in full, and use it for whatever purposes they wish. In fact, I would be delighted if someone were to copy this entire body of work. I encourage everyone to duplicate and mirror it in its entirety. I also encourage everyone to adapt and utilize the information in whatever manner they deem appropriate. No citation or other reference is requested or required. It would actually bring me great joy to see this information multiply exponentially and "go viral".
All content is free to all readers.
All support is deeply appreciated.



















































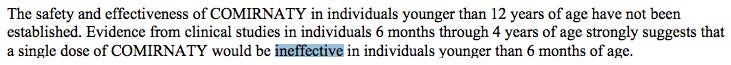












It is not a vaccine IT IS A BIOLOGICAL WEAPON!🥲
You are doing a truly sterling job putting all of this information together James. Thank you for your dedication in this fight. I am glad Dr Paul Alexander is also promoting your work.