An Open Letter to Robert F. Kennedy Jr.
The HHS Secretary is legally obligated to take immediate action to recall the Pfizer and Moderna mRNA injectables because they “present an imminent or substantial hazard to the public health.”
FOR COMPLETE DETAILS: NotSafeAndNotEffective.com
You may download the PDF version of the “Open Letter to Robert F. Kennedy Jr.” or scroll down to read the online text version.
In addition to reading the “Open Letter to Robert F. Kennedy Jr.,” please review the information below:
Dr. Jessica Rose
https://rumble.com/v4h2xwo-dr.-jessica-rose-phd-13-facts-on-covid-19-injections.html
Nicholas Hulscher, MPH
https://x.com/McCulloughFund/status/1872647597824844133
https://x.com/Inversionism/status/1873114320839778542
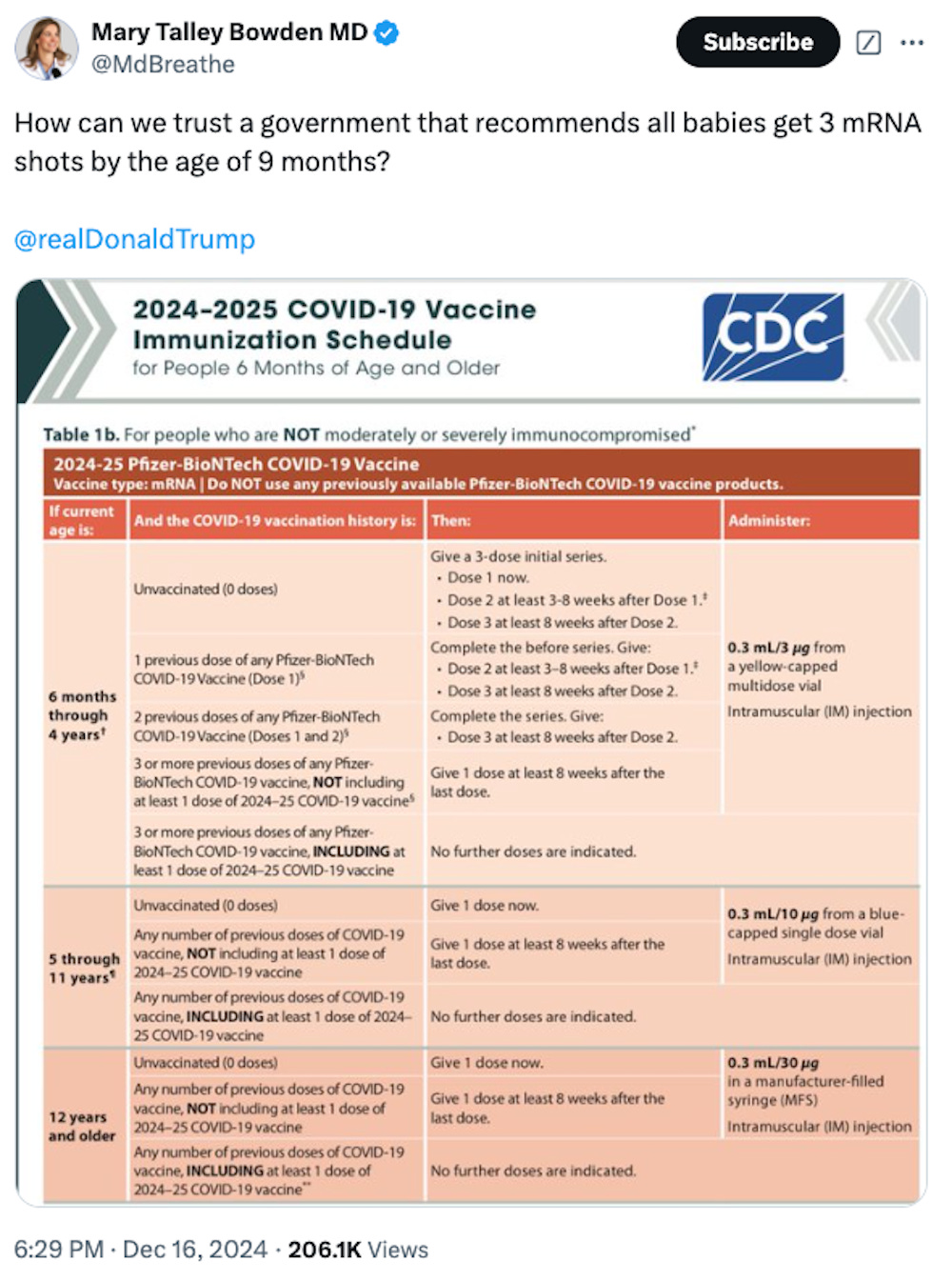
https://x.com/MdBreathe/status/1868845927676756375
https://childrenshealthdefense.org/defender/fda-authority-recall-covid-vaccines/
Robert F. Kennedy Jr.
President Trump has given me three instructions.
He wants the corruption and the conflicts out of the regulatory agencies.
He wants to return the agencies to the gold standard, empirically-based, evidence-based, science and medicine that they were once famous for.
And he wants to end the chronic disease epidemic with measurable impacts on a diminishment of chronic disease within two years.
- Robert F. Kennedy Jr.
https://edition.cnn.com/2024/11/14/politics/robert-f-kennedy-donald-trump-hhs/index.html
An Open Letter to President-Elect Donald Trump’s Nominee for Secretary of the Department of Health and Human Services, Robert F. Kennedy Jr.
December 29, 2024
Dear Robert F. Kennedy Jr.,
This open letter is written in support of your efforts to Make America Healthy Again.
In my humble opinion, you will NOT be able to Make America Healthy Again unless you convince President Donald J. Trump that Operation Warp Speed was a grave mistake.
The biological products licenses for the Pfizer and Moderna mRNA COVID-19 “vaccines” must be REVOKED, the mRNA injections must be RECALLED, they must be REMOVED from the children’s vaccine schedule and they must be taken off the market IMMEDIATELY.
As the Secretary of the Department of Health and Human Services, YOU will have both the lawful authority as well as an obligation to stop the carnage that these ill-conceived products have caused.
After your confirmation and IMMEDIATELY after taking the oath of office as the Secretary of the Department of Health and Human Services, I call upon you to exercise your authority and fulfill your obligations under the laws listed below to RECALL the Pfizer and Moderna mRNA “vaccines” because they present a documented, imminent and substantial hazard to the public health.
I also implore you to direct the FDA Commissioner to REVOKE the biological products licenses for Pfizer’s and Moderna’s “vaccine.” There are multiple reasons for which 21 USC § 601.5 enables the FDA to revoke the licenses for these products.
Please note the appropriate sections of the United States Code in Annex 2 attached to this letter.
Pfizer clearly failed to properly notify the FDA of a change in the manufacturing procedures from what has come to be known as “process 1” which was used to produce the biological products for the clinical trials. They switched to “process 2” in order to produce the biological products that have been injected into billions of people worldwide.
Both Pfizer’s and Moderna’s products have caused harm and death that far exceed the Bradford Hill criteria. Please watch the video below:
https://rumble.com/v4h2xwo-dr.-jessica-rose-phd-13-facts-on-covid-19-injections.html
Please remember that on May 16, 2021 you, along with Mary Holland and Dr. Meryl Nass, on behalf of your organization, Children’s Health Defense, submitted a Citizen Petition requesting that Janet Woodcock, the acting FDA Commissioner at the time, “revoke Emergency Use Authorizations for existing COVID vaccines and refrain from approving and licensing them.”
https://childrenshealthdefense.org/wp-content/uploads/FDA-2021-P-0460- 0001_attachment_1.pdf
- - - - -
Please review the blatantly inaccurate and deceptive communication that Peter Marks, the Director of the Center for Biologics Evaluation and Research, sent to Florida Surgeon General Joseph Ladapo on December 14, 2023:
https://www.fda.gov/media/174875/download
- - - - -
Please also be aware of these two letters from Senator Ron Johnson about the lack of transparency regarding the adverse events associated with the mRNA vaccines:
https://www.ronjohnson.senate.gov/services/files/00AAFB3D-72EE-475F-94D5- 66708B4AA86D
https://www.ronjohnson.senate.gov/services/files/FB6DDD42-4755-4FDC-BEE9- 50E402911E02
- - - - -
Supportive evidence is available here:
https://www.ijvtpr.com/index.php/IJVTPR/article/view/101/341 (Part 1) https://www.ijvtpr.com/index.php/IJVTPR/article/view/104/359 (Part 2)
and here:
https://NotSafeAndNotEffective.com
- - - - -
The current situation meets the criteria for a CLASS 1 recall:
Class I recall: a situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.
https://www.fda.gov/safety/industry-guidance-recalls/recalls-background-and-definitions
- - - - -
The Secretary of the Department of Health and Human Services has the authority to take action to protect the health of the American people when a biological product “presents an imminent or substantial hazard to the public health,” and is also OBLIGATED BY LAW TO TAKE ACTION:
“the Secretary [of the Department of Health and Human Services] SHALL issue an order immediately ordering the recall of such batch, lot, or other quantity of such product.”
- - - - -
The most effective way for you to help Make America Healthy Again is to do everything in your power to remove the mRNA injectable products from the marketplace.
Failure to remove the mRNA COVID-19 “vaccines” from the marketplace will give the FALSE impression that the mRNA platform is “safe and effective” and will leave the door wide open for future development of additional mRNA “vaccines” that may result in similarly horrific results.
If you fail to act IMMEDIATELY, then every adverse event, every disability and every death that is subsequently caused by the mRNA injections will occur due to YOUR failure to take immediate action.
I encourage you to take action upon this matter as a top priority as soon as you take the oath of office as the Secretary of the Department of Health and Human Services.
God bless you.
God Bless America.
God bless all the people of the world.
Sincerely,
James Roguski
310-619-3055
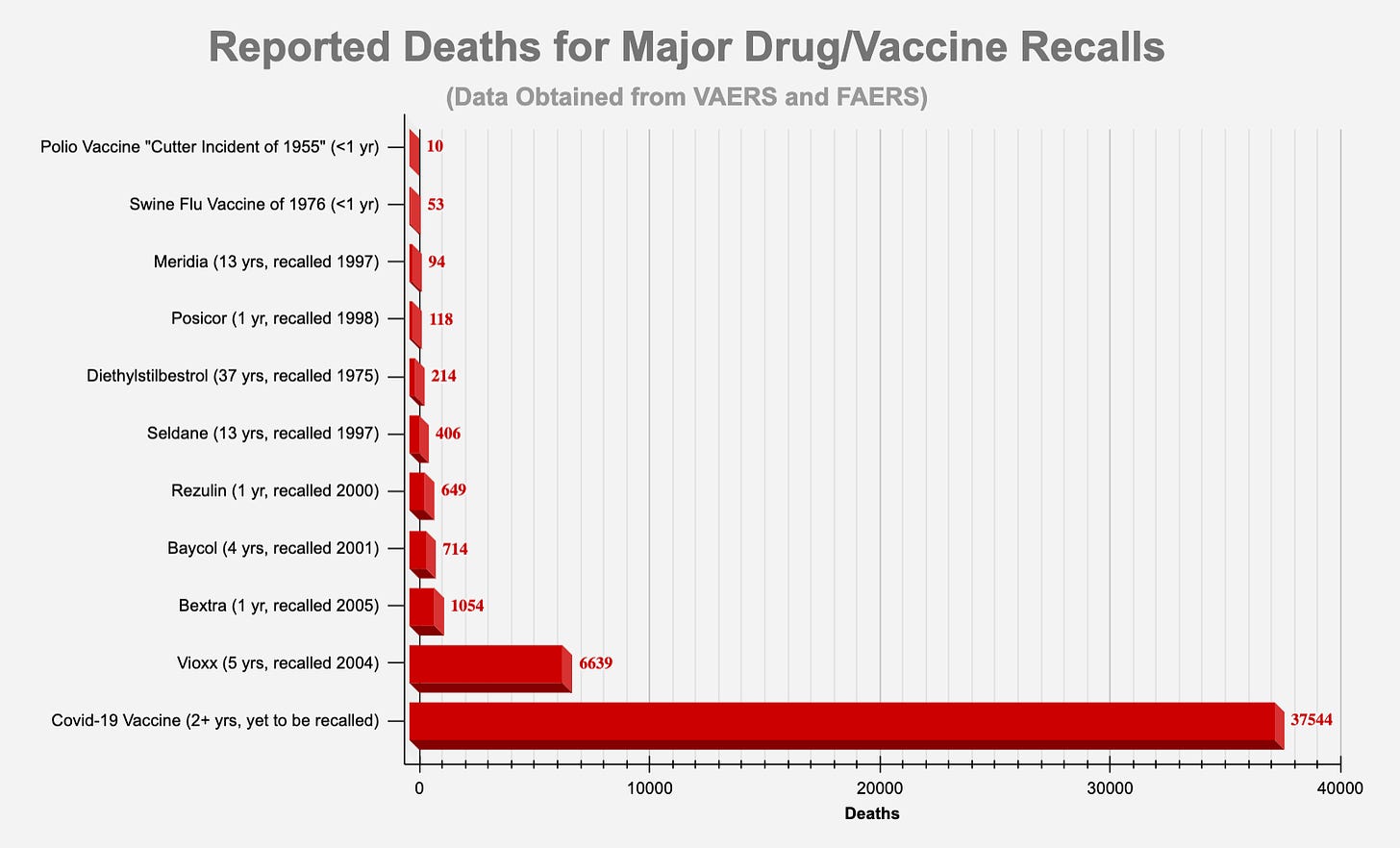
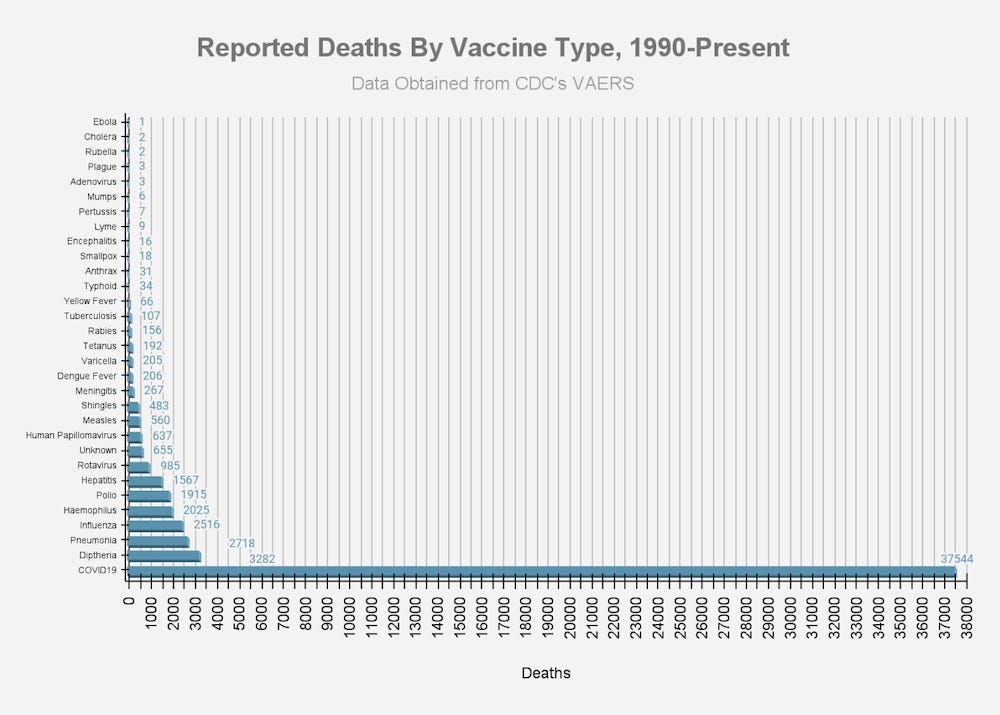
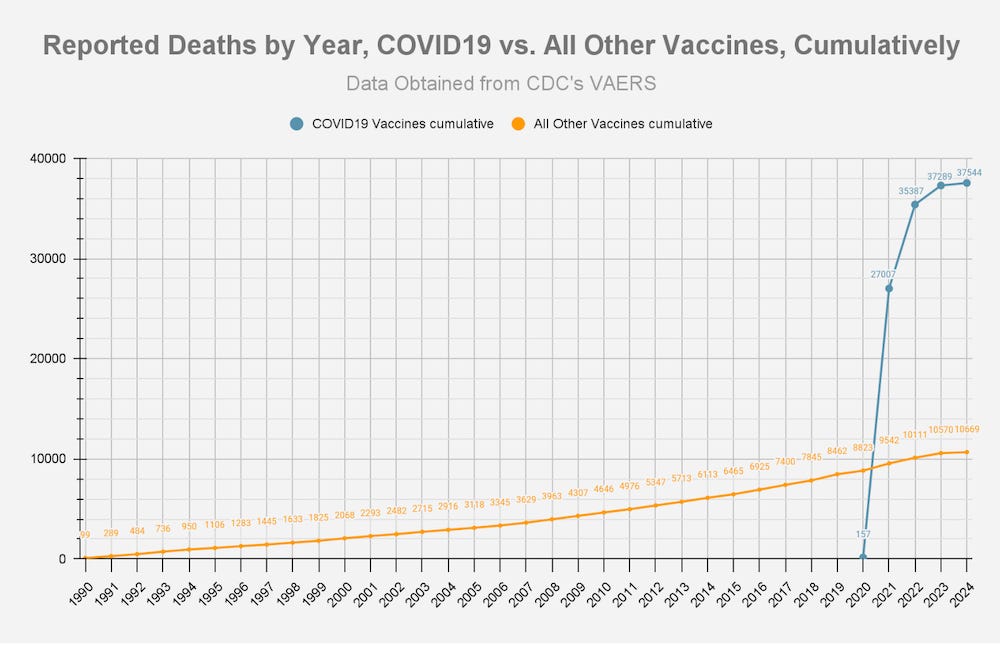
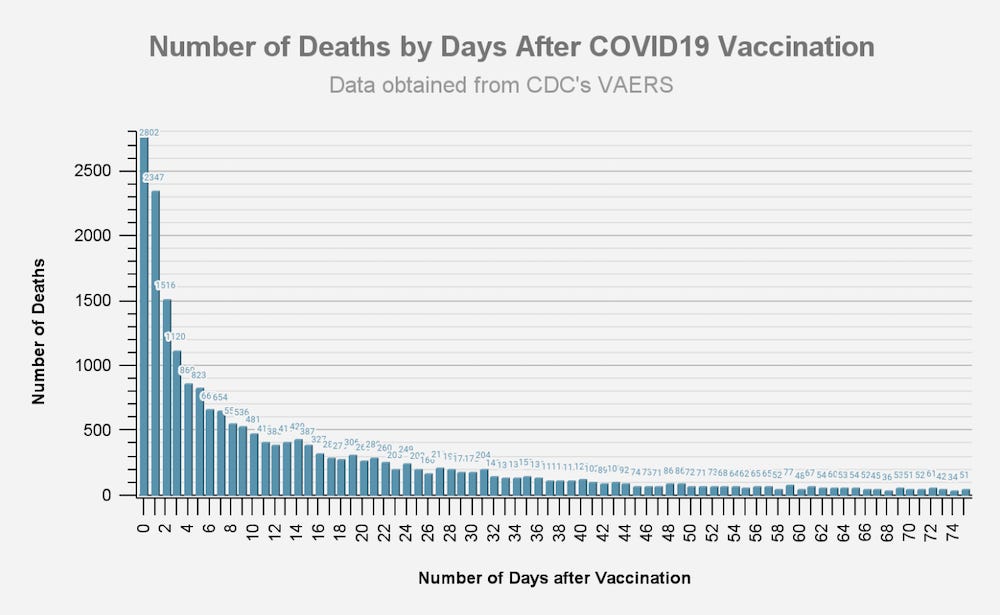
ANNEX 1: DOCUMENTATION OF HARM
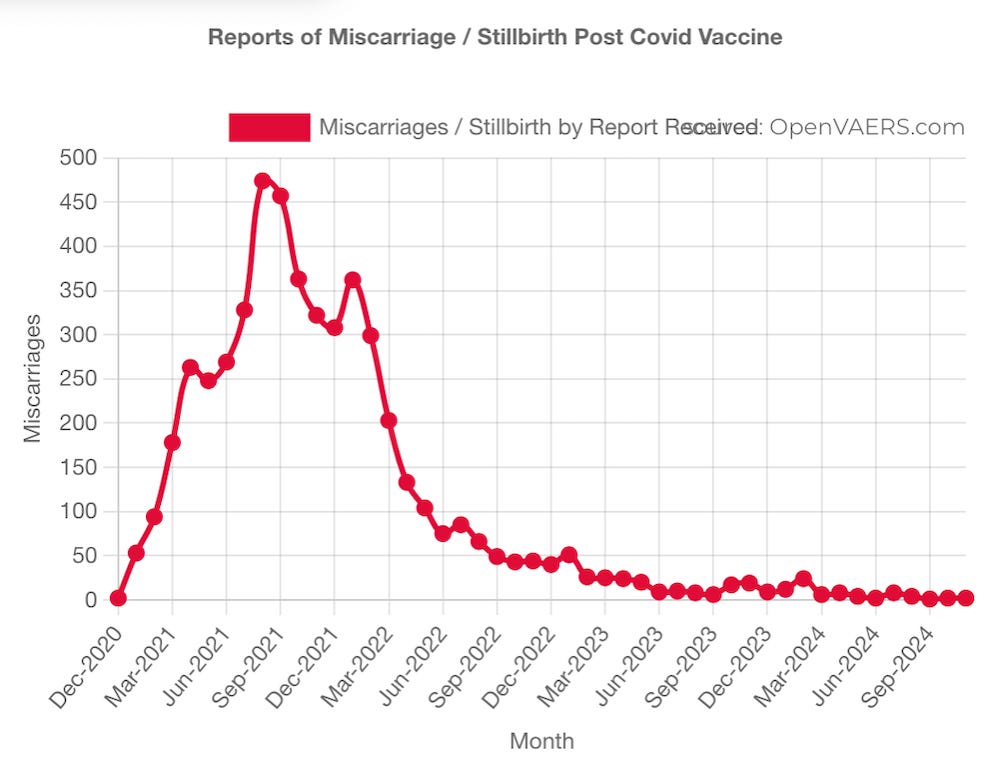
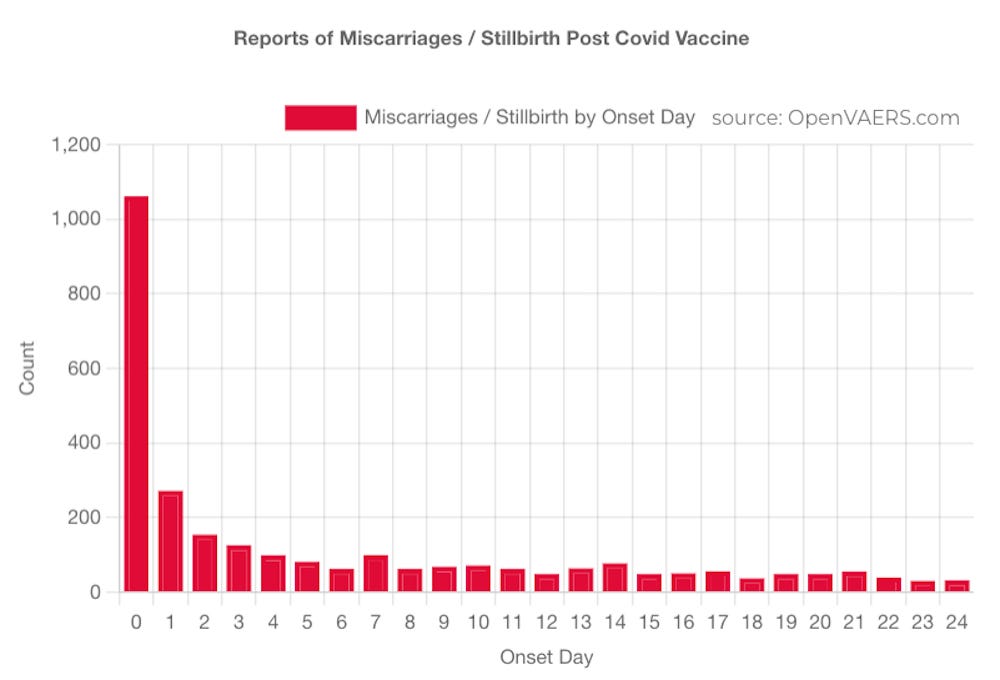
THOUSANDS OF REPORTS OF FETAL DEATHS THAT OCCURRED WITHIN HOURS OR DAYS OF THE mRNA INJECTIONS SHOULD BE FAR MORE THAN ENOUGH OF A “SAFETY SIGNAL” TO REQUIRE THESE DEADLY PRODUCTS TO BE TAKEN OFF THE MARKET.
https://www.openvaers.com/covid-data/reproductive-health
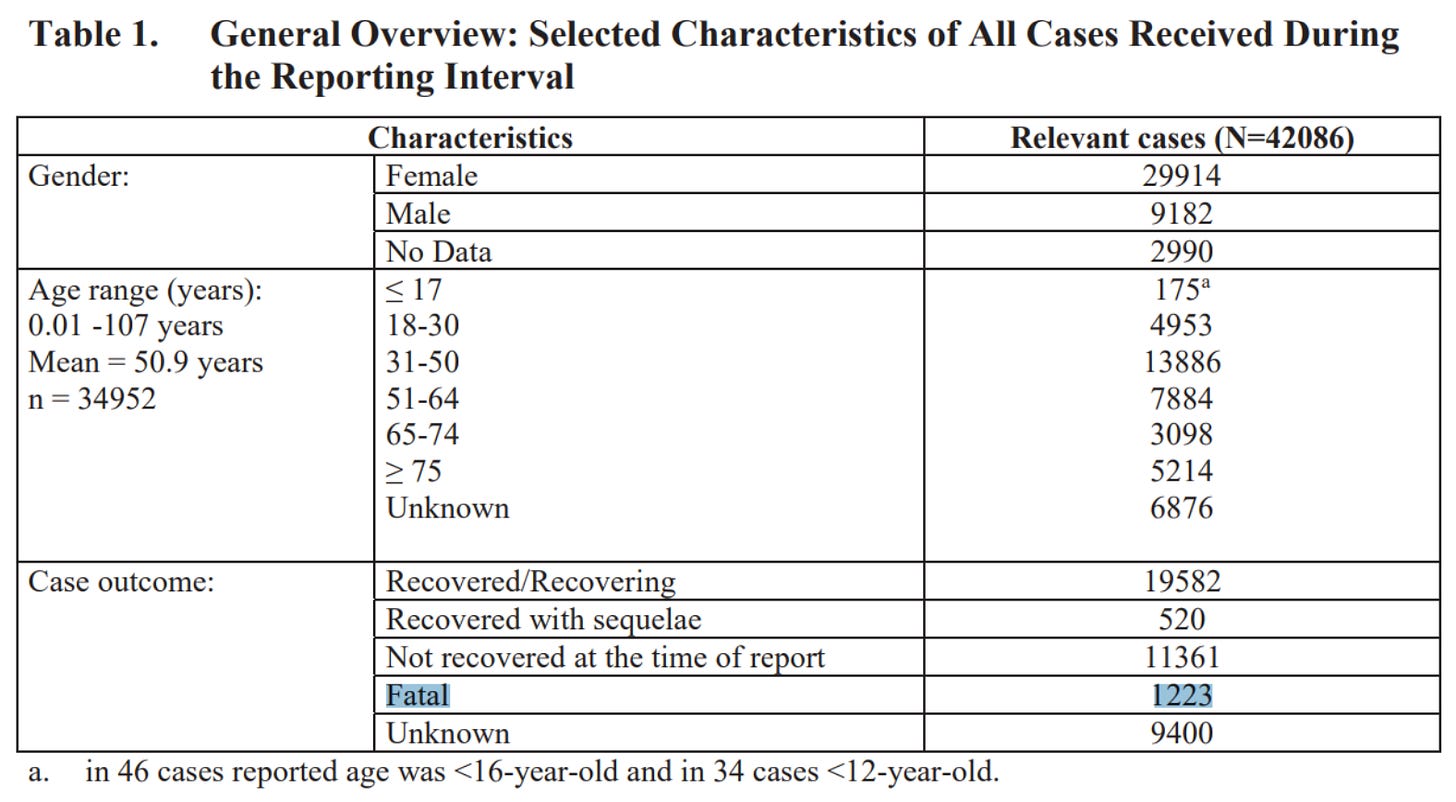
5.3.6 CUMULATIVE ANALYSIS OF POST-AUTHORIZATION ADVERSE EVENT REPORTS OF PF-07302048 (BNT162B2) RECEIVED THROUGH 28-FEB-2021
By February 28, 2021, it was known that the Pfizer “vaccine” alone had killed 1,223 people.
https://phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing- experience.pdf
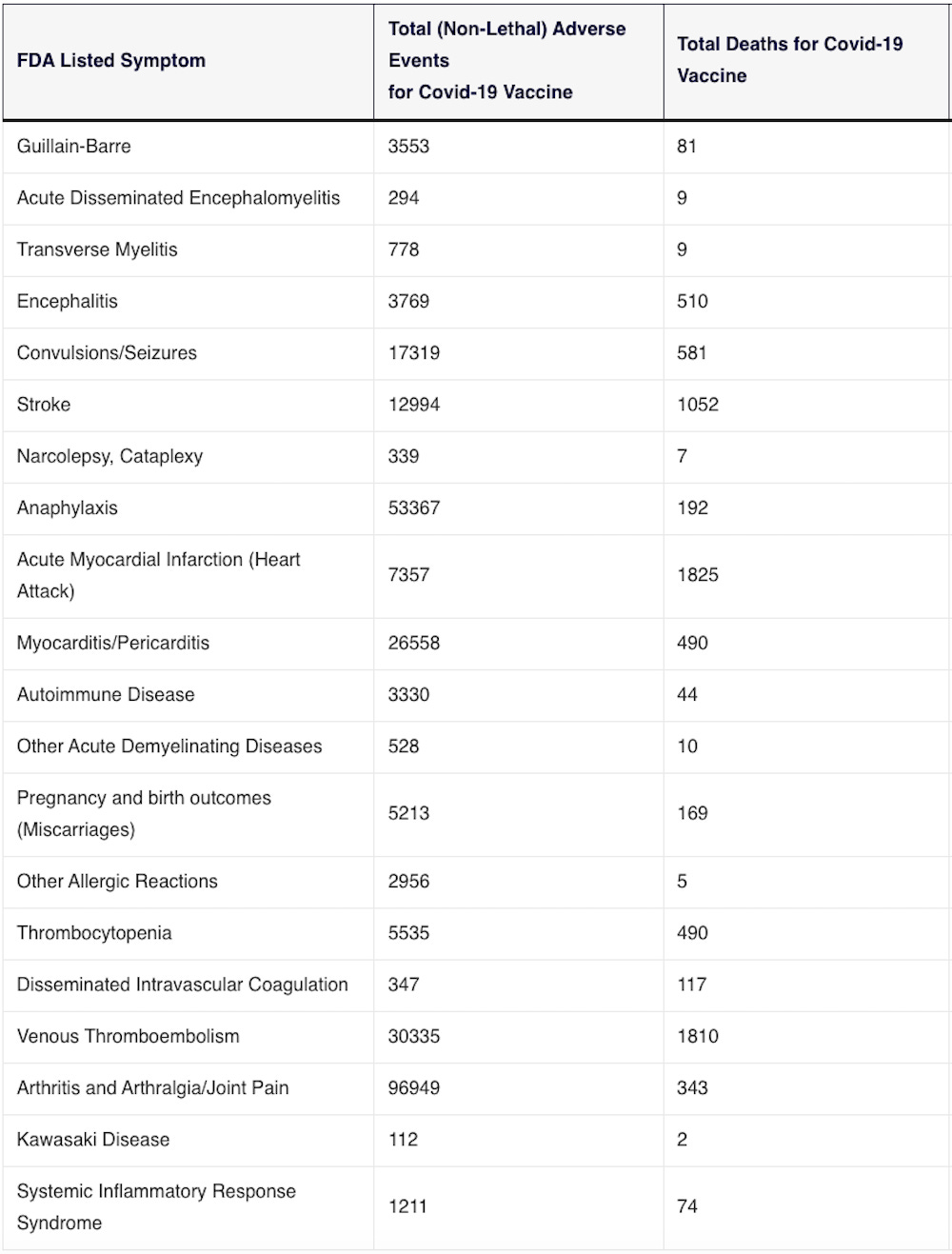
The slide below was taken from an FDA document from October 22, 2020 and provides a “Working list of possible adverse event outcomes” related to the COVID-19 vaccines.
“Vaccines and Related Biological Products Advisory Committee October 22, 2020 Meeting Presentation.”
https://www.fda.gov/media/143557/download (page 17)
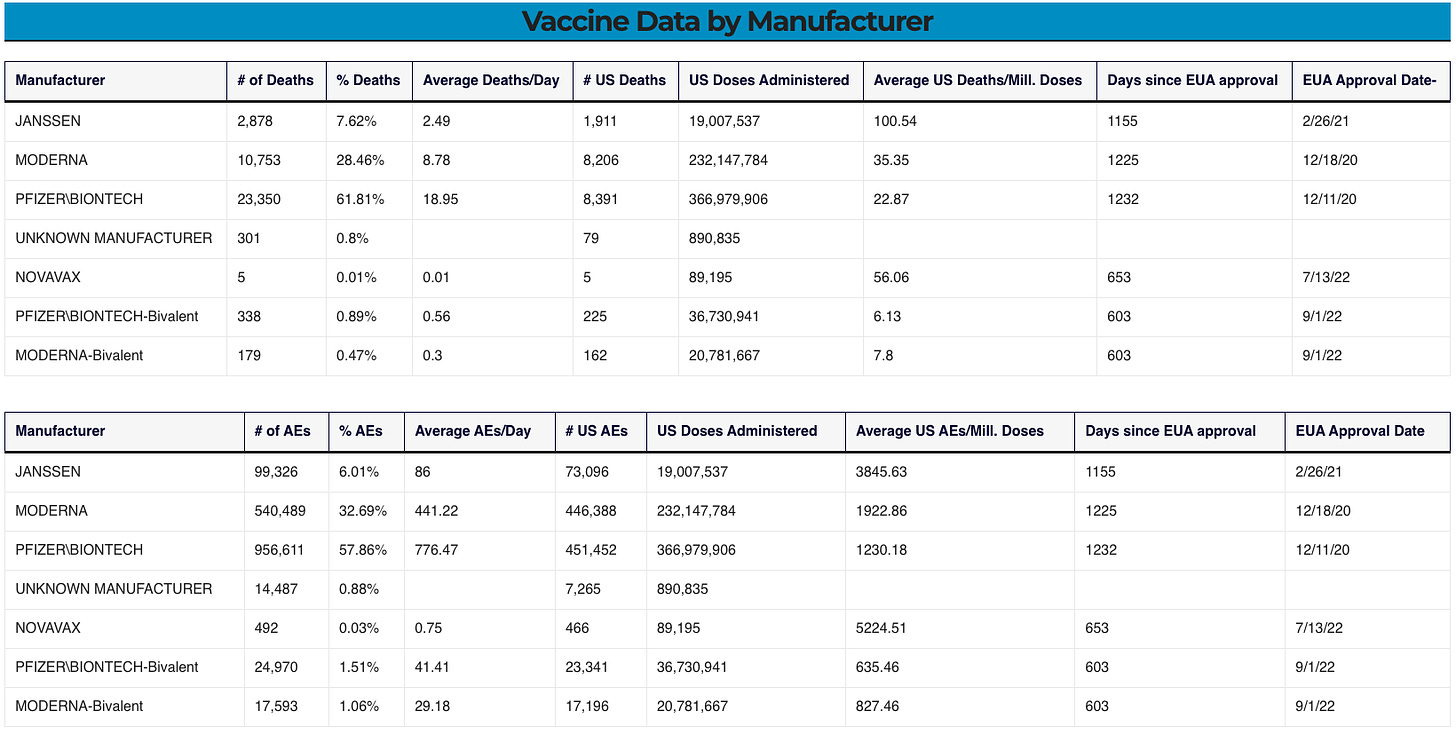
Please review the following table which lists the number of adverse events found in the VAERS data which match the “possible adverse event outcomes” listed above:
VAERS data
SOURCE: https://vaersanalysis.info/2024/05/03/vaers-summary-for-covid-19-vaccines-through-4-26-2024/
ANNEX 2: UNITED STATES CODE
42 USC §262 Regulation of biological products
(d) Recall of product presenting imminent hazard; violations
(1) Upon a determination that a batch, lot, or other quantity of a product licensed under this section presents an imminent or substantial hazard to the public health, the Secretary [of the Department of Health and Human Services] shall issue an order immediately ordering the recall of such batch, lot, or other quantity of such product. An order under this paragraph shall be issued in accordance with section 554 of title 5.
https://uscode.house.gov/view.xhtml?req=(title:42%20section:262%20
- - - - -
10 USC § 810.10 Revocation, suspension, or modification of authorization.
The Secretary may revoke, suspend, or modify a general or specific authorization:
(a) For any material false statement in an application for specific authorization or in any additional information submitted in its support;
(b) For failing to provide a report or for any material false statement in a report submitted pursuant to § 810.12;
(c) If any authorization governed by this part is subsequently determined by the Secretary to be inimical to the interest of the United States or otherwise no longer meets the legal criteria for approval; or
(d) Pursuant to section 129 of the Atomic Energy Act.
https://www.ecfr.gov/current/title-10/chapter-III/part-810/section-810.10
- - - - -
21 USC § 7.41 Health hazard evaluation and recall classification.
(a) An evaluation of the health hazard presented by a product being recalled or considered for recall will be conducted by an ad hoc committee of Food and Drug Administration scientists and will take into account, but need not be limited to, the following factors:
(1) Whether any disease or injuries have already occurred from the use of the product.
(b) On the basis of this determination, the Food and Drug Administration will assign the recall a classification, i.e., Class I, Class II, or Class III, to indicate the relative degree of health hazard of the product being recalled or considered for recall.
https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-7
- - - - -
21 USC § 314.150 Withdrawal of approval of an application or abbreviated application.
(a) The Food and Drug Administration will notify the applicant, and, if appropriate, all other persons who manufacture or distribute identical, related, or similar drug products as defined in §§ 310.6 and 314.151(a) of this chapter and for a new drug afford an opportunity for a hearing on a proposal to withdraw approval of the application or abbreviated new drug application under section 505(e) of the act and under the procedure in § 314.200, if any of the following apply:
(1) The Secretary of Health and Human Services has suspended the approval of the application or abbreviated application for a new drug on a finding that there is an imminent hazard to the public health. FDA will promptly afford the applicant an expedited hearing following summary suspension on a finding of imminent hazard to health.
(2) FDA finds:
(i) That clinical or other experience, tests, or other scientific data show that the drug is unsafe for use under the conditions of use upon the basis of which the application or abbreviated application was approved; or
(ii) That new evidence of clinical experience, not contained in the application or not available to FDA until after the application or abbreviated application was approved, or tests by new methods, or tests by methods not deemed reasonably applicable when the application or abbreviated application was approved, evaluated together with the evidence available when the application or abbreviated application was approved, reveal that the drug is not shown to be safe for use under the conditions of use upon the basis of which the application or abbreviated application was approved; or
(iii) Upon the basis of new information before FDA with respect to the drug, evaluated together with the evidence available when the application or abbreviated application was approved, that there is a lack of substantial evidence from adequate and well-controlled investigations as defined in § 314.126, that the drug will have the effect it is purported or represented to have under the conditions of use prescribed, recommended, or suggested in its labeling; or
(iv) That the application or abbreviated application contains any untrue statement of a material fact; or
(v) That the patent information prescribed by section 505(c) of the act was not submitted within 30 days after the receipt of written notice from FDA specifying the failure to submit such information;
https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-D/section-314.150
- - - - -
21 USC § 601.5 Revocation of license.
(a) A biologics license shall be revoked upon application of the manufacturer giving notice of intention to discontinue the manufacture of all products manufactured under such license or to discontinue the manufacture of a particular product for which a license is held and waiving an opportunity for a hearing on the matter.
(b)
(1) The Commissioner shall notify the licensed manufacturer of the intention to revoke the biologics license, setting forth the grounds for, and offering an opportunity for a hearing on the proposed revocation if the Commissioner finds any of the following:
(i) Authorized Food and Drug Administration employees after reasonable efforts have been unable to gain access to an establishment or a location for the purpose of carrying out the inspection required under § 600.21 of this chapter,
(ii) Manufacturing of products or of a product has been discontinued to an extent that a meaningful inspection or evaluation cannot be made,
(iii) The manufacturer has failed to report a change as required by § 601.12 of this chapter,
(iv) The establishment or any location thereof, or the product for which the license has been issued, fails to conform to the applicable standards established in the license and in this chapter designed to ensure the continued safety, purity, and potency of the manufactured product,
(v) The establishment or the manufacturing methods have been so changed as to require a new showing that the establishment or product meets the requirements established in this chapter in order to protect the public health, or
(vi) The licensed product is not safe and effective for all of its intended uses or is misbranded with respect to any such use.
(2) Except as provided in § 601.6 of this chapter, or in cases involving willfulness, the notification required in this paragraph shall provide a reasonable period for the licensed manufacturer to demonstrate or achieve compliance with the requirements of this chapter, before proceedings will be instituted for the revocation of the license. If compliance is not demonstrated or achieved and the licensed manufacturer does not waive the opportunity for a hearing, the Commissioner shall issue a notice of opportunity for hearing on the matter under § 12.21(b) of this chapter.
https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-601/subpart-A/section-601.5
- - - - -
21 USC § 601.12 Changes to an approved application.
(a) General.
(1) As provided by this section, an applicant must inform the Food and Drug Administration (FDA) (see mailing addresses in § 600.2 of this chapter) about each change in the product, production process, quality controls, equipment, facilities, responsible personnel, or labeling established in the approved license application(s).
(2) Before distributing a product made using a change, an applicant must assess the effects of the change and demonstrate through appropriate validation and/or other clinical and/or nonclinical laboratory studies the lack of adverse effect of the change on the identity, strength, quality, purity, or potency of the product as they may relate to the safety or effectiveness of the product.
(b) Changes requiring supplement submission and approval prior to distribution of the product made using the change (major changes).
(1) A supplement shall be submitted for any change in the product, production process, quality controls, equipment, facilities, or responsible personnel that has a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of the product as they may relate to the safety or effectiveness of the product.
https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-601/subpart-C/section-601.12
Many people and organizations around the world have been calling for the removal of the mRNA products from the market for quite a long time.
PLEASE HEED OUR CALL TO TAKE ACTION.
March 10, 2021
Urgent Open Letter from Doctors and Scientists to the European Medicines Agency regarding COVID-19 Vaccine Safety Concerns
We demand that approval for use of the gene-based vaccines be withdrawn until all the above issues have been properly addressed by the exercise of due diligence by the EMA.
May 16, 2021
Mary Holland, Robert F. Kennedy Jr. and Dr. Meryl Nass
Submitted a Citizen Petition requesting that Janet Woodcock, the acting FDA Commissioner at the time…
“…revoke Emergency Use Authorizations for existing COVID vaccines and refrain from approving and licensing them.”
https://www.regulations.gov/document/FDA-2021-P-0460-0001
https://childrenshealthdefense.org/wp-content/uploads/FDA-2021-P-0460-0001_attachment_1.pdf
https://childrenshealthdefense.org/defender/sign-petition-chd-fda-take-covid-vaccines-off-market/
June 30, 2021
James Roguski
https://www.bitchute.com/video/DAGbYvaTyNmq
September 24, 2021
Jonathan Weissman
Dear _ _ _ _ _,
I am writing today to inform you of the unacceptable risks in deploying the experimental COVID-19 genetic vaccines.
My fully independent, fully referenced biosecurity risk assessment thoroughly expounds upon all the risks documented across the fields of toxicology, molecular biology, virology, immunology and molecular, genomic and statistical epidemiology. It is hosted at alltherisks.com. I've also attached it to this email.
It highlights the exact pathologies associated with these genetic vaccines, explaining precisely how these experimental vaccines damage the body in devastating ways.
It also proves that, even prior to COVID-19, the strategy of mass vaccinating the population with non-sterilising vaccines targeting a highly mutable surface protein (spike) during a pandemic of a mutable RNA virus was already known to be a foolish, reckless and dangerous strategy.
These experimental genetic vaccines are unsafe, do not confer cross-protective immunity and will devastate our society's health, according to the academic literature and data we already have available.
We urgently need immediate public debate. I am absolutely willing to present all of this evidence personally to your organisation, in any capacity, under any reasonable set of conditions. The public deserves to be immediately informed of all the risks that we already know. Failure to immediately inform parents of all the risks their children would be exposed to is immoral, disgraceful and completely unacceptable. I do not believe our society would ever heal from such a betrayal by its leaders in politics and public health.
If you disagree that the public deserve to be immediately informed of all the risks, please justify your rationale.
Yours sincerely,
Jonathan Weissman BSc MSc
September 27, 2021
Dr. Jane Ruby
In the Pfizer COMIRNATY and Pfizer-BioNTech Covid 19 Vaccination Series package insert, (See Exhibit B), the label states that on December 11, 2020, during the randomized, placebo-controlled pivotal trial (the research design required for FDA approval), “participants were “unblinded to offer placebo participants COMIRNATY,” which in my expert opinion, immediately transformed the study (as the company itself indicated in its registry on ClinicalTrials.gov, NCT04368728) into a modified-open label, observational, variable dose trial with no informed consent as to the status change, the exact dosage, or full disclosure of ingredients and completely compromised the requisite data for license application and that should render the study data insufficient and inappropriate to file for or be considered for review for FDA approval. What resulted was the distribution of an incomplete marketing label out to the public. In my expert opinion this is an egregious and fraudulent misrepresentation of the Safe and Effective statements made to the public.
https://drjaneruby.com/wp-content/uploads/2023/12/Ruby-Affidavit_DTR_20210927_FINAL.pdf
November 29, 2021
World Council For Health
There is now more than enough evidence to declare the novel COVID-19 vaccines unsafe for use in humans. Victim testimonies and adverse reaction reporting systems have revealed millions of adverse reactions to the experimental vaccines, including life-changing injury and death.
The inoculations are capable of causing immeasurable harm to those who received them, with children being more likely to die from the Covid-19 vaccines than from actual SARS-CoV-2 infection.
World Council for Health anticipates that unprecedented humanitarian efforts will be essential to assist the people harmed by this global vaccination experiment, due to the known and unknown harms.
The World Council for Health demands an end to this crisis and hereby declares it illegal and unlawful for anyone to participate, directly or indirectly, in this harmful experimental vaccination programme. The World Council for Health declares individuals, governments, and other corporations will be held liable for their involvement.
https://www.worldcouncilforhealth.org/covid-19-vaccines/
https://wchweb.b-cdn.net/web/downloads/Article-Files/cease-and-desist/cease-and-desist-1-2.pdf
August 20, 2022
https://rumble.com/v1ees0f-right-docs-of-history-strike-back-stop-the-shots.html
StopTheShots.net
#StopTheShots
In the video above, over 40 doctors speak out in support of #StopTheShots
The doctors are listed below in the order of appearance in the video.
Dr. Marivic Villa
Dr. Michael Uphues
Dr. Kat Lindley
Dr. Bob Apter
Dr. Mary Talley Bowden
Dr. Paul Alexander
Dr. Peter McCullough
Dr. Avani Gupta
Dr. Bruce Boros
Dr. Myhuong Nguyen
Dr. Ben Marble
Dr. Pierre Kory
Dr. Chris Shoemaker
Dr. Grams
Dr. James A. Thorp
Dr. Sally Priester
Dr. Lynnell Lowry
Dr. Chris Hall
Dr. Fynn
Dr Rob Lowry
Dr. Moon
Dr. Steve Latulippe
Dr. Molly Rutherford
Dr. Calvin Blount
Dr. Erin Greer
Dr. Bryan Tyson
Dr. Mollie James
Dr. Terry Lakin
Dr. Claire Zengerle
Dr. Anthony
Dr. Angie Farella
Dr. Aaron Williams
Dr. Debra Viglione
Dr. Avery Brinkley
Dr. Neelu
Dr. Villa
Dr. John Witcher
Dr. Sonya Naryshkin
Dr. David Vella
Dr. Sigoloff
Dr. Bryan Ardis
Dr. Judy Mikovitz
Dr. Ellapen Robert Rapiti:
https://rumble.com/v1iv58h-stop-the-jab-and-save-lives.html
March 21, 2023
Association of American Physicians and Surgeons Statement Calling for Moratorium on COVID-19 Shot Mandates and Genetic Injections
1. COVID 19 injections are under Emergency Use Authorization and must be considered experimental. Informed consent is a bedrock principle of medical ethics, yet millions of people have taken COVID-19 injections under duress.
2. The long-term effects of the novel mRNA or DNA technology and the lipid nanoparticles involved in their administration – including carcinogenesis, mutagenesis, autoimmune phenomena, and impairment of fertility – cannot possibly be known.
3. There are numerous safety signals, including excess sudden deaths, that would in the past have prompted immediate withdrawal of vaccines or drugs from the market.
4. The expected intensive, sophisticated investigations of reported adverse effects associated with COVID-19 vaccination, including myocarditis, pericarditis, paralysis, thromboembolism, menstrual abnormalities, and unusual cancers, have not been undertaken.
5. COVID-19 genetic injections have not been shown in randomized, controlled trials to be effective in preventing infection, transmission, hospitalization or death.
6. In children who have virtually zero likelihood of death from COVID, there is no evidence of benefit exceeding risks for these products.
7. Regulatory agencies are corrupted by conflicts of interest, lack of transparency, and lack of accountability.
8. Vaccine-injured patients have little if any expectation of compensation, and manufacturers are shielded from liability. This liability protection must be ended.
9. All mandates, including requirements for school attendance or work, should immediately be withdrawn.
10. COVID-19 genetic injections should be withdrawn from the market.
https://aapsonline.org/aaps-statement-calling-for-moratorium-on-covid-19-injections-and-mandates/
Starting date unknown
Americans For Health Freedom
We declare, and the data confirms that COVID-19 experimental genetic therapy injections must end. All COVID-19 and other modified mRNA “vaccines” must be immediately discontinued.
We demand that Covid-19 vaccines be removed from the pediatric vaccine schedule.
https://www.americansforhealthfreedom.org/
I pledge to call for the COVID shots to be pulled off the market.
January 3, 2024
Florida State Surgeon General Calls for Halt in the Use of COVID-19 mRNA Vaccines
Florida State Surgeon General Dr. Joseph A. Ladapo has released the following statement:
“The FDA’s response does not provide data or evidence that the DNA integration assessments they recommended themselves have been performed. Instead, they pointed to genotoxicity studies – which are inadequate assessments for DNA integration risk. In addition, they obfuscated the difference between the SV40 promoter/enhancer and SV40 proteins, two elements that are distinct.
DNA integration poses a unique and elevated risk to human health and to the integrity of the human genome, including the risk that DNA integrated into sperm or egg gametes could be passed onto offspring of mRNA COVID-19 vaccine recipients. If the risks of DNA integration have not been assessed for mRNA COVID-19 vaccines, these vaccines are not appropriate for use in human beings.
Providers concerned about patient health risks associated with COVID-19 should prioritize patient access to non-mRNA COVID-19 vaccines and treatment. It is my hope that, in regard to COVID-19, the FDA will one day seriously consider its regulatory responsibility to protect human health, including the integrity of the human genome.”
https://www.floridahealth.gov/newsroom/2024/01/20240103-halt-use-covid19-mrna-vaccines.pr.html
June 29, 2024
COVID-19 Modified mRNA “Vaccines”: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio-Pharmaceutical Complex, Part 1
We urge governments to endorse and enforce a global moratorium on these modmRNA products and the lipid nanoparticle delivery platform, unless and until all relevant questions pertaining to causality, residual DNA, and aberrant protein production are resolved.
https://www.ijvtpr.com/index.php/IJVTPR/article/view/101/341 (Part 1)
July 3, 2024
The Hope Accord
THE IMMEDIATE SUSPENSION OF THE COVID-19 mRNA VACCINE PRODUCTS
A growing body of evidence suggests that the widespread rollout of the novel Covid-19 mRNA vaccine products is contributing to an alarming rise in disability and excess deaths.
The association observed between the vaccine rollout and these concerning trends is now supported by additional significant findings. These include the discovery of plausible biological mechanisms of harm demonstrated in laboratory and autopsy studies, as well as high rates of adverse events seen in randomised clinical trials and national surveillance programs. Altogether, these observations indicate a causal link.
This new technology was granted emergency use authorisation to address a situation that no longer exists. Going forward, the burden of proof falls on those still advocating for these products to compellingly demonstrate that they aren’t resulting in net harm. Until such evidence is presented, regulators should suspend their use as a matter of standard medical precaution.https://TheHopeAccord.org
August 16, 2024
COVID-19 Modified mRNA “Vaccines”: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio-Pharmaceutical Complex, Part 2
Since we currently have no discernible way to predict serious adverse event occurrences, it seems criminal to allow this Russian roulette schema to persist in the context of injecting infants and young children, especially by coercion or mandate.
We once again urge governments worldwide to mandate a moratorium on the modmRNA products until proper safety and toxicological studies are performed and openly shared with the scientific community.
https://www.ijvtpr.com/index.php/IJVTPR/article/view/104/359
September 25, 2024
I urge you to take immediate action to suspend these vaccines pending further investigation and comprehensive, independent testing.
November 8, 2024
Idaho
https://www.bitchute.com/video/zhOzUMpVzQcO
Winter, 2024
For any other medicinal product, the regulatory submission would have been considered incomplete and most probably rejected. Therefore, a moratorium on the use of Pfizer/BioNTech COVID-19 vaccines and boosters should be enacted at minimum, but ideally, they should be removed from the market and their use in humans should be stopped. It should be the responsibility of the pharmaceutical industry, not independent scientists, to determine whether a medical intervention is safe. Based upon Pfizer/BioNTech’s data, safety of their COVID-19 modRNA vaccine has not been proven.
December 3, 2024
Conclusion: Our results raise grave concerns regarding the safety of the BNT162b2 vaccine and call for an immediate halt of all RNA biologicals unless these concerns can be dispelled.
December 22, 2024
Nicolas Hulscher, MPH
Immediate action to remove these deadly injections from the market should be a top priority for the incoming administration. Failure to do so would likely cause an irreversible loss of trust in our government and represent a grave disservice to the American people.
https://petermcculloughmd.substack.com/p/study-reveals-covid-19-vaccines-have
January 17, 2025
READ THE ARTICLE:
https://standforhealthfreedom.com/stop-the-shots/
SEND AN EMAIL:
https://standforhealthfreedom.com/actions/stop-the-shots/
James Roguski
310-619-3055
JamesRoguski.substack.com/archive
ControlBloodSugarNaturally.com
I claim no copyright of any kind whatsoever, over any of my work, ever. Everyone is encouraged to copy any and all of it, in part, or in full, and use it for whatever purposes they wish. In fact, I would be delighted if someone were to copy this entire body of work. I encourage everyone to duplicate and mirror it in its entirety. I also encourage everyone to adapt and utilize the information in whatever manner they deem appropriate. No citation or other reference is requested or required. It would actually bring me great joy to see this information multiply exponentially and "go viral".
All content is free to all readers.
All support is deeply appreciated.






















































If you cannot be on Trump's team to clean out the medical control swamp team, MAHA, then be on God's team by putting on the whole armour of God. Pray that Trump's team can only do good, and stop the Covid-19 RNA or DNA injections. They were not 95% safe or effective. That was false advertising, promotions to the medical consumers and the Tax Payers.
And to investigate the current stock pile of vials to test how many in the city's current shipment were Placeboes and what percentage was hot, toxic stuff in the other vials that caused vaccine injuries or deaths.
Pray that the justice team, the Coroners will report how a person died by being vaxxed within the last 14 days of life, or had died many months after their last vaccination, or an unvaxxed person, died who was never ever Covid-19 vaxxed or flu vaxxed, and how that unvaxxed person truly died.
Fantastic post! Thank you! I’m trying to red pill as many people as possible and this is overwhelmingly easy with this volume of irrefutable data.